Otology
Vol. 45: Issue 4 - August 2025
The vascular anatomy of the internal auditory canal. A reappraisal for function preservation surgery
Abstract
Objectives. Partial or complete loss of function of the 7th and 8th nerves is a common occurrence in surgery of the cerebello-pontine angle, with the causal mechanism being considered the mechanical insult on nerves and the critical loss of blood supply to nerves and labyrinth. Preventing a vascular loss requires both soft surgery and knowledge of hitherto disregarded details of vascular anatomy. The goal of this paper is to provide the missing picture of descriptive and surgical anatomy.
Methods. Data of vascular anatomy were obtained from: (i) a group of 100 injected temporal bones submitted to microdissection; (ii) a group of 30 sectioned temporal bones; (iii) a group of 30 cases who underwent surgery for small vestibular schwannoma.
Results. A detailed picture was obtained concerning position and course of the anterior inferior cerebellar artery, origin and course of internal auditory artery (IAA) and its branches. Handling and cleavage of IAA from nerves was also explored.
Conclusions. The role of blood supply in function preservation of 7th-8th nerves comes from preclinical studies on animals as well as anatomical studies and clinical-surgical observations in humans. Updated knowledge of vascular anatomy is expected to provide surgeons with hitherto disregarded details and contribute to function preservation.
Introduction
Critical reduction of blood supply can be considered as a cause of functional loss of the facial or cochlear nerve during surgery in the internal auditory canal (IAC) and cerebello-pontine angle (CPA). The injury may be on the arteries, veins and vascular network of nerves, dura and arachnoid, and also causes peripheral ischaemia to cochlea spiral ganglion and sensory cells. Knowledge of vascular anatomy dating back to past authors 1-4 and more recent ones 5-14 still holds true, but lacks crucial details for microsurgery. Over the years, 7th and 8th cranial nerves preservation surgery 15 with technical tools like high-resolution microscopes, endoscopes and exoscopes have provided better visualisation of anatomical details that elucidated the classical pictures. The present study investigated details of the vascular anatomy integrating them in the classical descriptive anatomy, as is necessary for function preservation surgery.
Materials and methods
This study was conducted in accordance with the Anatomical QUality Assurance (AQUA) Guidelines for Reporting Original Anatomical Studies 16, when applicable (see checklist: Supplementary S1). The study included findings from 3 databases. The first database was composed by a continuous series of 100 fresh, human temporal bones submitted to vascular injection of colored silicone and studied with microdissection. The second database included a series of 30 fresh bones submitted to both injection of vessels and osmic acid staining of the labyrinth and studied with 1 mm thick, cleared sections technique, with a number of cases previously studied with microdissection. Data had been in part reported in articles on arteries of the CPA 9, blood supply of the petrous bone 10 and vestibular labyrinth 11. The third source of data was a surgical series of 30 cases of small vestibular schwannomas (VS) operated on via a translabyrinthine or retrosigmoid approach, which provided intraoperative pictures of vascularisation of nerves in the auditory canal and CPA. For the readers’ convenience, all anatomical figures represent the right side.
Vascular anatomy
The IAC, acoustic porus and adjacent CPA were the object of study. The vessels were the anterior inferior cerebellar artery (AICA), posterior-inferior cerebellar artery (PICA), internal auditory artery (IAA) and venous system draining the IAC. The description of arteries and veins was divided in 3 sections as follows: (i) position and course of AICA, or PICA, in the CPA prospecting the acoustic porus, (ii) AICA with initial portion of IAA in CPA, (iii) IAA in IAC. A paragraph on venous drainage of the IAC into the cerebral and bone circulation concludes this section.
Results
Arteries of the cerebello-pontine angle and the internal auditory canal
AICA (OR PICA) IN THE CPA
A cerebellar arterial loop (AICA/PICA) lies either inside (40%; Fig. 1A,B and Cover figure) or at the porus (27%, Fig. 2) of the IAC or in the CPA close to the porus (33%) 13. It supplies one or 2 IAAs, the subarcuate artery and several small branches entering the IAC, which are also called internal auditory arteries because of their function and distribution. This loop is the main trunk or a collateral of AICA in 80% of cases, of the accessory AICA in 17%, or a branch of the PICA in 3%. The complex loop-IAA-subarcuate artery exhibits a variety of arrangements from a plain, C-shaped loop to 2-3 tortuous, winding loops, or a straight course. In 10% of our cases a sized (Fig. 2A) or more commonly one or 2 slender (Fig. 2A) recurrent branch arises from AICA and runs back along the 8th nerve to the cerebellum. In 12% of cases the loop becomes adherent, or enclosed, with its convex portion or the recurrent limb to the dura of the subarcuate fossa, or it is closely held to the fossa by a short subarcuate artery 13.
AICA AND IAA
The IAA can be single or double, herein called IAA-I and IAA-II. They occur with equal rate and have a consistent course in the IAC and variable course in the CPA from their parent vessel to the 8th nerve depending on the position of AICA with the 7th-8th nerve bundle in CPA or IAC (see Cover figure). The IAAs originate from the loop outside of the IAC in 60% of cases (Fig. 2C,D), inside and close to the opening of the IAC in 40% (Figs. 1,5), or else the IAA-I inside and IAA-II outside the IAC (Fig. 1A,B). The single IAA and IAA-I arise mostly from the proximal limb (Fig. 1A, Fig. 2) or the convexity of AICA, more often in CPA than in IAC, and occasionally fused in a single branch with the subarcuate artery from which it soon divides, either in CPA or inside the IAC (Fig. 1A). The IAA-II arises more often from the proximal limb of the AICA (Fig. 1A, Fig. 3A), but may also arise from the distal limb of the AICA (Fig. 1B).
Both single and double IAA run from their parent artery across the CPA or IAC, depending on their origin, to the lower rim of IAC and from there they have 2 possible courses. The artery passes cranially between the 7th and 8th nerves, rarely in front of the 7th nerve, to reach the anterior-superior side of the 8th nerve. In the second instance, the IAA remains on the floor and below the 8th nerve. Rarely, both IAAs have a reverse origin from the distal limb of AICA (Fig. 1B).
In summary, there are 2 pictures of the IAA course as follows: (i) single IAA on the anterior-superior side of the 8th nerve; (ii) 2 IAAs, in which the first arising artery is the IAA-II that lies on the floor of the IAC; the IAA-I lies on the anterior-superior side of the 8th nerve. This order occasionally is reversed (Fig. 1B).
The detailed course of the IAA, single or double condition, from origin to the 8th nerve depends on the position of the loop with respect to the nerves at porus or IAC, and in CPA, as follows:
- AICA loop lying at porus or IAC and:
- – below the nerves (Fig. 2B,C,D): IAA, single or I, runs to the inferior-anterior side of the 7th nerve, thereon it crosses anteriorly and reaches the superior side of the 8th nerve within the proximal third of IAC (Fig. 2D); the IAA-II runs directly to the inferior-posterior side of the 8th nerve;
- – between the nerves (Fig. 1A, Fig. 2A): the IAA, single or I, runs along the anterior then superior side of the 8th nerve (Fig. 1A, Fig. 2B), or directly on the superior side of the 8th nerve; the IAA-II crosses to the inferior-posterior side of the 8th nerve;
- – above the nerves (Fig. 1B): the IAA, single or I, runs directly on the superior side of the 8th nerve; the IAA-II crosses to the inferior-posterior side of the 8th nerve.
- AICA lying in the CPA (Fig. 2D, Fig. 3A) with a looping or straight course: the IAA, single or I, runs directly to the 8th nerve on the anterior-superior side; the IAA-II on the inferior-posterior side.
The IAA-II presents a consistent course. It runs from the AICA to the inferior rim of the porus to the inferior side of the 8th nerve.
IAA IN THE IAC (COVER FIGURE, FIG. 2, FIG. 4, FIG. 5)
Both IAAs supply numerous branches to the nerves, dura and petrous bone all along their course. Also, a variable number of single, slender arteries arising from AICA loop enter the IAC and distribute to nerves, dura and bone. The arachnoid mesh that lies between nerves and dura carries a rich network of small arteries and veins that supply them. There are 2 possible positions of the IAA inside the IAC: the anterior-superior surface of the 8th nerve and the inferior-posterior surface of the 8th nerve. The former position is more commonly taken by the single IAA or, with the double IAA condition, by the IAA-I, and the second position by the IAA-II (Fig. 1A, Fig. 2B, Fig. 3A).
SINGLE IAA AND IAA-I
They have identical course within the canal. The artery runs in the proximal third of the canal between the anterior surface of the 8th nerve and anterior wall of the canal, and fairly close to, sometimes in contact with, the floor. In the middle third of the canal, it obliquely crosses the anterior side of the nerve and rests on the superior side of the same nerve just prior to its division, being covered by the facial nerve. The artery continues its course on the superior side of the cochlear nerve and then divides in its labyrinthine branches. The latter origin and initial course occur within the triangular area bounded by the diverging cochlear and vestibular nerves and at a lower level than the crista trasversalis.
The superior vestibular artery branches off first. It runs a tortuous, coiled path in the cochlea-vestibular triangle and curls cranially at the end of the canal, where it passes over the crista trasversalis and enters the osseous canal of the superior vestibular nerve. It rests at the lower limit of the wall and, adhering to the nerve, enters the vestibule by following the utricular nerve. The main stem of the IAA, called common cochlear artery, continues a tortuous course in the cochleo-vestibular triangle arriving to the depth of the canal, where it divides into cochlear artery and vestibulo-cochlear artery. This division can also occur just after the origin of the superior vestibular artery, and from there on the 2 arteries run a tortuous course in the cochleo-vestibular triangle. The cochlear artery becomes adherent to the lower aspect of the cochlear nerve and, crossing the canal depth, follows the same spiral course as the nerve.
The vestibulo-cochlear artery runs close to the canal floor following the inferior vestibular nerve and enters a separate osseous channel at a point between the saccular nerve and cochlear nerve bundles for the basal end of the cochlea. The artery divides with a T pattern into the basal cochlear artery and inferior vestibular artery, the division also taking place either within the bone of the inferior quadrant or at the vestibule medial wall.
THE IAA-II OF DOUBLE IAAS
The IAA-II, in the double IAA condition, runs to the floor of IAC and below the 8th nerve; it originates the vestibulo-cochlear artery which takes the course described above.
Veins of the internal auditory canal
The labyrinth has a consistent and plain venous drainage, while the IAC presents a variegated venous system. The labyrinth has a double venous drainage: the cochlear vein and the vestibular vein.
The IAC has 2 routes of venous drainage, the dura-bone path and the CPA path. In the first, veins collect on the nerves and cross the cerebrospinal fluid (CSF) to the canal dura and thereon they enter the bone where they merge and enter the inferior petrosal sinus. The CPA path involves one or more veins on the nerves that exit the porus and merge in a collecting trunk up to the petrosal vein that ends in the superior petrosal sinus (Cover figure, Fig. 2A, Fig. 4, Fig. 5).
Areas of potential injury of the internal auditory arteries and veins
The IAA, both single and IAA-I, from AICA to the 8th nerve has no defensive tissue but the soft arachnoid mesh, thus making a strip of potential injury that extends from AICA to anterior-inferior side of the 8th nerve. Thereon, the IAA lies along the anterior then superior side of the 8th nerve for the mid-third of IAC, and then it lies in the V space of the vestibular and cochlear nerve getting up to the fundus. The mentioned first portion from origin to 8th nerve and the further one on the 8th nerve constitute the potential areas of risk of injury during tumour dissection in VS surgery. The IAA-II has a similar risk of injury from the parent artery to the 8th nerve, while further on along the floor and inferior aspect of the 8th nerve it is protected by the nerve. The extensive anastomoses among supplying vessels on the network (Figs. 4, 6) of nerves and dura in the IAC seem to be a notable feature for vicarious supply in the case of injury of a branch.
The veins are fragile and difficult to preserve. The posterior vestibular vein is at risk of being hit during drilling of meatotomy on the posterior wall of the IAC, or at lifting off the dura from the area of drilling. There is no evidence that vein injury with integrity of vestibular aqueduct can cause hearing loss. Damage to the cochlear vein is harmful to hearing and can occur if drilling widely extends between the floor of the IAC and jugular bulb.
Discussion
This paper aimes at integrating the classical picture of vascular anatomy of the 7th and 8th cranial nerves as given by the classical and later authors with important details that are necessary for functional surgery as recently obtained from both descriptive anatomy studies and intraoperative data, which are paramount.
The IAA, the diameter of which is 0.1 to 0.2 mm, can be torn, compressed, or constricted. Interruption of the IAA, both experimentally and in surgery, is known to cause deafness. The effects of temporary or permanent injury to IAA on facial and vestibulo-cochlear function have been investigated in several preclinical studies in animals and humans 17-24.
Knowledge of the course of arteries in the IAC and CPA, and their various relationships with the cochlear and facial nerves, as outlined in the present study, represents a preliminary step in performing functional surgery and developing successful preservation strategies. Nevertheless, the intraoperative microsurgical handling of vessels and nerves remains a high-risk procedure, as the mechanisms underlying functional loss are not yet fully understood.
Pathophysiology of cranial nerve damage in VS surgery
Inquiring for causes and modalities of functional loss of the 7th-8th nerves in VS surgery involved looking for a strict temporal correlation between unequivocal surgical acts and the functional loss. Direct mechanical insult and the mediated vascular insult as well as the subsequent function loss seem to be the events specifically involved with surgery. Stretching, compression and heat shock may frequently occur during surgical dissection manoeuvres, putting nerves at risk of further physical injury, in addition to direct tear. The cumulative effect of different, sequential acts can make up an important cause of loss.
A change of the normal physiologic context of nerves and vessels occurs in surgery. Collapse of the brain at opening of the dura and CSF draining off involves tension on nerves due to brain displacement, as well as tension on the arterial loop and especially to the IAA. The collapse of nerves bundle in IAC tears down the arterioles and veins running between nerves and dura, thus causing a potential loss of blood supply or vasospasm of the whole system. The CSF replaced with irrigation of saline solution may not be a favourable change. While the acute loss is a minority of cases, a slow loss is a more frequent event and hardly liable to be associated with a surgical act. The small size of auditory arteries, their involvement with the tumour and surgical manoeuvres seem to point to acute or delayed ischaemia as the cause of postoperative deafness. Similarly, loss of blood supply to the spiral ganglion 25 can be a cause of failed cochlear implant despite a well-preserved cochlear nerve.
The logical step would therefore be to use a technique respecting the IAA, keeping the tumour dissection away from the artery and adopting a sort of surgical restraint all along its course from AICA to porus, as well as in IAC. To prevent direct damage by handling the artery, a feasible step is to strictly advance on the tumour surface without invading the adjacent tissues, i.e. arachnoid and connective layer on nerve, without disrupting the nerve-arachnoid connection. The first critical point occurs at the tumour’s posterior pole, where the dissection may hit the IAA that runs from the parent vessel to the nerve. Here, it is convenient to identify the thin arachnoid layer, lift it off the tumour and dissect in between the tumour and arachnoid. At the distal third of IAC, dissection should closely follow the tumour and stay away from the lower quadrants, where the labyrinthine branches emerge on nerves surface.
When the IAA is visible, it can be separated all along its course by following the thin connective layer lying between artery and tumour. Of note, a mechanical insult can have a different effect both on site and distally. On site, a direct tear on a nerve, like neurotmesis or axonotmesis, and on a vessel with tear or vasoconstriction, and distally as secondary ischaemia on nerve, spiral ganglion and sensory cells.
Is the position of the vessels modified by the tumour? In VS, both the 7th and 8th nerves are displaced and flattened on the anterior wall of the IAC and the arteries follow them. Exceptions to this picture require more data. It appears that the surgical technique for preservation of bloody supply is at the beginning of its development and that more studies are necessary.
Conclusions
Knowledge of the vascular anatomy in the IAC and CPA is paramount to strategies for functional preservation of the 7-8th cranial nerves in VS surgery. Loss of function of facial and cochlear nerve in surgery for VS, or other lesions, can be acute or slow, caused by mechanical trauma either direct or mediated by loss of blood supply through tears, vasoconstrictions and depletion of the capillary bed. It is difficult to differentiate the 2 events, mechanic and ischaemic, in the case of slow loss, and probably both occur as a result of the insult.
Preventing the loss during surgery can be promoted by better knowledge of details of vascular anatomy in CPA and IAC, as well as by an appropriate dissection technique that closely follows the tumour surface and observes a sort of surgical restraint along the course of IAA and veins. A soft microsurgical dissection to identify the artery, followed by careful dissection along the connective layer between the artery and tumour, as well as preservation of the vascular supply within the arachnoid tissue enveloping the nerves, constitute preliminary steps for a successful surgical technique.
Acknowledgements
The authors thank Ms. Alison Garside for reviewing the English language.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
AM: conceptualization, methodology, validation, formal analysis, investigation, resources, writing-original draft; SC: methodology, software, investigation, resources, data curation, writing-original draft; writing - review & editing, visualization; LF: writing-original draft, writing-review & editing; LA: writing-original draft, writing-review & editing; DdA: conceptualization, writing-review & editing, supervision; EZ: conceptualization, investigation, writing-original draft, writing-review & editing, supervision.
Ethical consideration
As our study is based on archival material and anatomical specimens previously dissected for other studies, ethical approval was not required. The study was conducted in accordance with the principles of the Helsinki Declaration, and data were handled in compliance with Italian privacy and data laws, as well as the internal rules for anatomical studies at the Otolaryngology Section of Padua University (Italy).
History
Received: February 13, 2025
Accepted: March 9, 2025
Figures and tables
Figure 1. A) The 7th and 8th nerves were dissociated to show the intermediate AICA looping inside the IAC. The IAA-I arises from proximal limb, the IAA-II from cerebello-subarcuate branch. Two recurrent additional arteries lie on the 8th nerve and run to cerebellum; B) Large AICA looping inside canal and supplying IAA-I and IAA-II from distal limb, subarcuate artery from proximal limb. *: proximal limb of AICA; +: cerebello-subarcuate artery; x: subarcuate artery; O: internal auditory artery I; Φ: internal auditory artery II; H: recurrent branches to cerebellum.
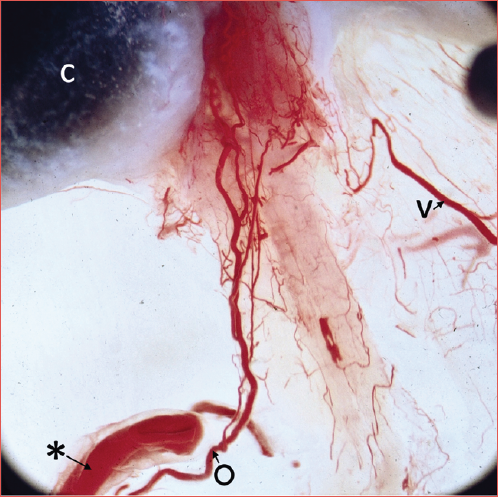
Figure 2. A) AICA looping inside the IAC. An internal auditory vein leaves the 8th nerve and joins the superior petrosal sinus. A single IAA branches off from proximal limb of the AICA, runs anterior to the 7th nerve and becomes visible inside the IAC on the 8th nerve (*: proximal limb of AICA; O: internal auditory artery; H: recurrent branches to cerebellum; v: internal auditory vein; 7: facial nerve; 8: eighth nerve); B) 7th cranial nerve raised: AICA lying at the porus, in intermediate position between the 7th and 8th nerves. IAA-I arising from proximal limb of AICA. A recurrent cerebello-subarcuate branch arising from the proximal limb supplies the IAA- II and subarcuate artery (*: AICA; O: internal auditory artery I; Φ: internal auditory artery II; +: cerebello-subarcuate artery; x: subarcuate artery; 7: facial nerve; 8: eighth nerve); C) AICA at the porus lying on vertical plane and anterior-inferior to nerves. Single IAA arising from proximal limb, subarcuate artery from the distal one. A sizeable recurrent branch loops back to cerebellum (*: proximal limb of AICA; #: recurrent branch from AICA to cerebellum; x: subarcuate artery; O: internal auditory artery; 7: facial nerve; 8: eighth cranial nerve); D) Large AICA looping below the 7th-8th nerves in the CPA. The single IAA branches off from proximal limb, the subarcuate artery from the distal limb (*AICA; x: subarcuate artery; O: internal auditory artery; 7: facial nerve; 8 eighth nerve; H: recurrent branches to the cerebellum).
Figure 3. A) The 7th and 8th cranial nerves lifted up to show the AICA, which lies in CPA and bifurcates in a recurrent limb to cerebellum and a cerebello-subarcuate artery; IAA-I arising from the recurrent branch, and IAA-II arising from the cerebello-subarcuate branch, which is held to the subarcuate fossa by the subarcuate artery; B) Injected and microdissected temporal bone. Bone was drilled off the internal auditory canal, the dura was opened and the 7th nerve was lifted up. The AICA loop supplies in sequence: a common branch to the dura and 8th nerve, the IAA-I which branches off in the artery for the superior vestibular ganglion, superior vestibular artery and common trunk of the cochlear and cochleo-vestibular artery. *: proximal limb of the AICA; O: internal auditory artery I; Φ: internal auditory artery II; +: cerebello-subarcuate artery; 7: seventh nerve; 8: eighth nerve; x: subarcuate artery; #: distal limb of AICA.
Figure 4. Detail of Cover figure. Injected, 1 mm thick, cleared section showing the vascular network on nerves of IAC supplied by branches of IAAs. 1: artery for geniculate ganglion; 2: superior vestibular artery; 3: common trunk of cochlear artery and vestibulo-cochlear artery; c: cochlea; v: vein.
Figure 5. Schematic representation of the arteries of the IAC, in the case of single (A) or double (B) IAA. In the double IAA condition, the vestibulo-cochlear artery of the IAA is the IAA-II, while the IAA-I remains with the superior-vestibular artery and cochlear artery.
Figure 6. Intraoperative pictures. A) Small vestibular schwannoma: intraoperative view of the IAA entering the IAC; B) IAA entering IAC and running between the 7th and 8th nerves. A small vestibular schwannoma is lifted up. S: vestibular schwannoma; O: internal auditory artery; 7: facial nerve; 8: eighth nerve.
References
- Eichler O. Anatomische Untersuchungen über die Wege des Blutstromes im menschlichen. Abhandlungen der Königlichen Sächsischen Gesellschaft der Wissenschaften. 1892;31:311-349.
- Siebenmann F. Die Blutgefässe im Labyrinthe des menschlichen Ohres. Published online 1894.
- Konaschko P. Die Arteria auditiva interna des Menschen und ihre Labyrinthäste. Z Anat Entwicklungsgesch. 1927;83:241-268. doi:https://doi.org/10.1007/BF02117937
- Lelli G. Comportamento dell’arteria uditiva interna e dei suoi rami labirintici nell’uomo. Z Anat Entwicklungsgesch. 1939;110:48-80. doi:https://doi.org/10.1007/BF02118213
- Guerrier Y, Villaceque G. Origine et comportement des artères cérébelleuse moyenne et auditive interne. CR Ass Anat. 1949;36:377-382.
- Nager G. Origins and relations of the internal auditory artery and the subarcuate artery. Ann Otol Rhinol Laryngol. 1954;63:51-61. doi:https://doi.org/10.1177/000348945406300104
- Axelsson A. The vascular anatomy of the cochlea in the guinea pig and in man. Acta Otolaryngol. Published online 1968.
- Fisch U. Surgical anatomy of the arterial system of the internal auditory canal in man. Rev Laryngol Otol Rhinol (Bord). 1968;89:659-671.
- Mazzoni A. Internal auditory canal arterial relations at the porus acusticus. Ann Otol Rhinol Laryngol. 1969;78:797-814. doi:https://doi.org/10.1177/000348946907800413
- Mazzoni A. The subarcuate artery in man. Laryngoscope. 1970;80:69-79. doi:https://doi.org/10.1288/00005537-197001000-00006
- Mazzoni A. The vascular anatomy of the vestibular labyrinth in man. Acta Otolaryngol. 1990;110:1-83. doi:https://doi.org/10.3109/00016489009121137
- Kim H, Kim Y, Park I. Variability of the surgical anatomy of the neurovascular complex of the cerebellopontine angle. Ann Otol Rhinol Laryngol. 1990;99(4 Pt 1):288-296. doi:https://doi.org/10.1177/000348949009900408
- Salgado-Lopez L, Leonel L, Aydin S. Surgical anatomy of the labyrinthine and subarcuate arteries and clinical implications. World Neurosurg. 2020;141:E880-E887. doi:https://doi.org/10.1016/j.wneu.2020.06.083
- Dos Santos J, Musso F, Mayer W. Descriptive and topographical analysis of the labyrinthine artery in human fetuses. Anat Sci Int. 2020;95:374-380. doi:https://doi.org/10.1007/s12565-020-00531-5
- Zanoletti E, Concheri S, Tealdo G. Early surgery and definitive cure in small sporadic vestibular schwannoma. Acta Otorhinolaryngol Ital. 2022;42:481-486. doi:https://doi.org/10.14639/0392-100X-N2322
- Tomaszewski K, Henry B, Kumar Ramakrishnan P. Development of the Anatomical Quality Assurance (AQUA) checklist: guidelines for reporting original anatomical studies. Clin Anat. 2017;30:14-20. doi:https://doi.org/10.1002/ca.22800
- Mom T, Avan P, Bonfils P. A model of cochlear function assessment during reversible ischemia in the Mongolian gerbil. Brain Res Protoc. 1999;4:249-257. doi:https://doi.org/10.1016/S1385-299X(99)00026-4
- Mom T, Telischi F, Martin G. Vasospasm of the internal auditory artery: significance in cerebellopontine angle surgery. Am J Otol. 2000;21:735-742.
- Franz L, Marioni G, Mazzoni A. Contemporary perspectives in pathophysiology of facial nerve damage in oto-neurological and skull base surgical procedures: a narrative review. J Clin Med. 2023;12. doi:https://doi.org/10.3390/jcm12216788
- Bendella H, Brackmann D, Goldbrunner R. Nerve crush but not displacement-induced stretch of the intra-arachnoidal facial nerve promotes facial palsy after cerebellopontine angle surgery. Exp Brain Res. 2016;234:2905-2913. doi:https://doi.org/10.1007/s00221-016-4692-7
- Sunderland S. Stretch-compression neuropathy. Clin Exp Neurol. 1981;18:1-13.
- Greiman M, Lusk R. Pressure-induced modifications of the acoustic nerve Part II: auditory brain stem responses. Am J Otolaryngol. 1991;12:12-19. doi:https://doi.org/10.1016/0196-0709(91)90068-Q
- Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40:1-10. doi:https://doi.org/10.1097/00006123-199701000-00001
- Hostettler I, Jayashankar N, Bikis C. Clinical studies and pre-clinical animal models on facial nerve preservation, reconstruction, and regeneration following cerebellopontine angle tumor surgery – A systematic review and future perspectives. Front Bioeng Biotechnol. 2021;9. doi:https://doi.org/10.3389/fbioe.2021.659413
- Mei X, Glueckert R, Schrott-Fischer A. Vascular supply of the human spiral ganglion: novel three-dimensional analysis using synchrotron phase-contrast imaging and histology. Sci Rep. 2020;10. doi:https://doi.org/10.1038/s41598-020-62653-0
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 961 times
- PDF downloaded - 189 times



