Head and neck
Vol. 45: 111TH CONGRESS SIOECHCF - OFFICIAL REPORT 2025
Margins in major salivary gland surgery: clinical and pathological criteria for defining margins and their implications on the choice of multimodal therapies. A systematic review
Abstract
Objective. Major salivary gland malignancies (MSGM) are a rare and heterogeneous group of tumours accounting for 1-5% of all head and neck cancers. When feasible, surgical removal with negative margins is the preferred treatment, reserving adjuvant radiotherapy for adverse clinicopathological features such as high-grade, advanced-stage, extranodal extension, lympho-vascular invasion, perineural invasion, and positive margins. This systematic review aims to evaluate the current literature on the definition of negative and close margins for MSGM, their impact on locoregional recurrence (LRR), disease-free (DFS), and overall survival (OS), and their implications in the choice of multimodal therapies.
Methods. An online search of articles published between 2004 and 2024 was carried out using PubMed via a PICO search strategy for qualitative questions and written following the PRISMA statement guidelines. The following parameters were evaluated: definition of free and close margins, and their impact on local control.
Results. The initial search yielded 158 articles. Following the application of inclusion and exclusion criteria, 30 full-text publications were reviewed. All studies were retrospective. A total of 15,985 patients who underwent surgery were considered. Margin involvement ranged widely among the studies from 14.3% to 65.4%. Five out of 30 studies reported no data about association between margins and LRR, DFS, and OS. Twenty of 25 studies reported a significant correlation between positive margins and oncological outcomes regardless of the histological types, while 5 focused on high-stage cancers or more aggressive histotypes and described no association between margin status and oncological outcomes. Nine of 30 studies described close margins in the absence of a univocal definition of threshold for close vs. negative margins. Most studies did not report a significant correlation between close margins and oncological outcomes.
Conclusions. Surgical resection achieving negative margins is recommended for MSGM. Positive margin is widely considered an adverse clinicopathological feature and performing adjuvant radiotherapy has documented survival benefits. A consensus involving a definition of close margin is missing, although further treatment is not recommended, preferring a watch-and-wait approach in presence of close margins.
Introduction
Major salivary gland malignancies (MSGM) are rare neoplasms accounting for 1-5% of all head and neck cancers 1 and include a wide spectrum of histological features with a variety of biologic behaviours 2. MSGM diagnosis is crucial for the application of appropriate treatment, although symptoms suggestive of malignancy, such as skin infiltration, rapid tumour growth, pain, infiltration of the surrounding structures or neck metastasis, are described in a small percentage of patients 3. Ultrasound, magnetic resonance, computed tomography and fine needle aspiration cytology represent routine preoperative exams necessary to correctly evaluate a salivary gland mass. Among major salivary glands, submandibular gland cancers are the rarest, accounting for 5-15% of all salivary neoplasms, and are associated with a significantly higher malignancy rate and worse prognosis than parotid tumours 4. Many of the 24 histotypes described contain subtypes with specific features that further increase the heterogeneity of such tumours. Despite the rarity and variety of histologic features of MSGM, several tumour characteristics have been studied to predict the evolution of the pathology and its appropriate treatment, including margin status.
Complete tumour resection with clear margins is the main goal of curative therapy 5,6. However, specific anatomical characteristics of the salivary glands and the frequent proximity of the tumour to crucial structures such as the facial nerve, make removal of MSGM with adequate tumour-free resection margins a challenging task for the surgeon. In addition, diagnosis of nerve involvement typically requires its sacrifice, and differentiating between benign and malignant tumours on frozen sections may be challenging 7. Probably due to the above-mentioned reasons, salivary cancers have higher rates of positive margins compared to other head and neck cancer sites. Finally, surgical margins defined as “close” represent a suboptimally defined and poorly understood group that may be ambiguous and not adequately defined.
Given the rarity and histological heterogeneity of such diseases, a systematic review with meta-analysis of the literature regarding the definition and prognostic significance of surgical resection margins is rather difficult to perform. The purpose of this systematic review is to analyse the existing literature on surgery for MSGM and discuss the prognostic significance of positive or negative margins, the definition of close margins, and implications on the implementation of multimodal therapies.
Materials and methods
Search strategy and information sources
An online computerised search of articles published between 2004 and 2024 was performed using PubMed via a PICO search strategy for qualitative questions and written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines 1.
Study selection and data extraction
The search was conducted using the following query: “major salivary gland” AND “tumour” AND “margin” NOT “lymphoma”. Inclusion criteria for abstract selection were as follows: original articles written in English, published after January 1st, 2004, involving MSGM, including data on resection margins, and at least 5 patients.
Studies not describing any surgical resection margins, which did not include an abstract, and case reports or case series with fewer than 5 patients, meta-analyses or systematic reviews, were excluded. The search was conducted in July 2024 by 2 of the authors (GP and VM) who independently screened titles and abstracts and subsequently discussed disagreements. When agreement was reached, the article was selected. The same authors screened the full texts of the studies selected and included articles that met inclusion criteria. Once again, the 2 authors discussed disagreements after reading the full text of all articles.
In the systematic review the following parameters were evaluated: definition of free and close margins, and impact of surgical margins on local control.
Results
According to the search criteria, 158 articles were found in the PubMed database. After analysing titles and abstracts, 41 were included for full-text review. Out of these, 11 were excluded for the above-mentioned exclusion criteria. In the end, a total of 30 articles were considered in our review. All studies were retrospective. A flowchart of the study selection process is shown in Figure 1. Two studies 9,10 used the same series and only one was considered. Data on margin status, local recurrence, and oncological outcomes are illustrated in Table I.
A total of 15,985 patients who underwent surgery for MSGM were considered, with a study sample size ranging from 19 to 4,431 patients. The recruitment period ranged from 1979 to 2020.
The primary tumour site was the parotid gland in 13,619 patients (85.2%), the submandibular in 1,948 (12.4%), the sublingual in 88 (0.5%), and was unspecified in 330 cases (2.1%). Tumour histology was as follows: 7,661 mucoepidermoid carcinoma (MEC) (48%), 3,136 adenoid cystic carcinoma (AdCC) (19.6%), 1,350 adenocarcinoma (AC) (8.4%), 1,142 acinic cell carcinoma (ACC) (7.1%), 527 squamous cell carcinoma (SCC) (3.3%), 382 salivary duct carcinoma (SDC) (2.4%), 284 oncocytic carcinoma (OC) (1.8%), 247 large cell undifferentiated carcinoma (LCUC) (1.5%), 154 carcinosarcoma (CS) (1%), 144 carcinoma ex-pleomorphic adenoma (CEPA) (0.9%), and 958 other tumours (6%).
Among patients considered in this analysis, 5,642 (35.3%) had positive margins. The rate ranged from 14.3% to 65.4% in different series. Five of 30 studies reported no data about the association between margins and loco-regional recurrence (LRR), disease-free (DFS), and overall survival (OS). Twenty studies reported a direct correlation between positive margins and oncological outcomes regardless of the histological types, whereas 5 studies, which focused generally on high-stage cancers or more aggressive histotypes, described no association between the two.
Nine of 30 studies 11-19 described close margins in 779 patients (4.9%) and in 3 other studies the positive and close margins were considered and analysed without distinction between the two 9,20,21. In 6 of 12 patients in this group, a cutoff between cut tissue edge and tumour was not defined 9,10,17-19,21, whereas in the remaining 6 studies it varied from 0.5 to 5 mm 11-16. In 4 studies it was less than 1 mm 11,12,15,16.
Discussion
Positive surgical margins are generally considered an adverse clinicopathological feature such as high-grade, advanced-stage, pathologic extranodal extension, lympho-vascular and perineural invasion. Surgical resection achieving negative margins is strictly recommended 10,12,22, even if consensus involving a definition of safe surgical margins (clinical margins) is missing. Obtaining tumour-free margins may be challenging given the anatomical complexity of this area, especially when trying to preserve adjacent structures. Regarding the parotid, facial nerve preservation is recommended in patients with intact preoperative function when a dissection plane can be created between the tumour and the nerve. However, resection of many parotid tumours often results in close or positive surgical margins due to the relationship between the tumour and facial nerve. Thus, partial removal of the affected gland in the early-stage and selected cases can be considered 23.
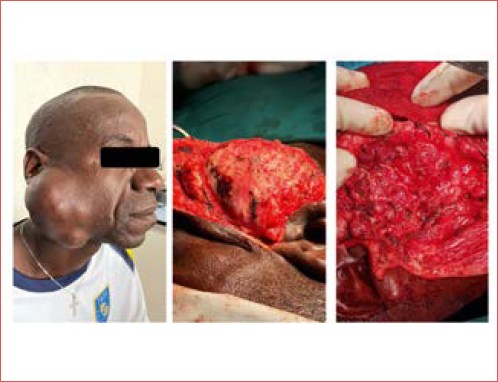
Salivary cancers have higher rates of positive margins compared to other head and neck cancer sites. In our analysis, 35.3% of patients reported positive margins with a range between 14.3% and 65.4%. The highest rate of positive margins was found in studies which mainly reported advanced-stage tumours (Cover figure). Patel 24 reported 65.4% positive margins considering cT4b MSGM and Tanvetyanon 19 reported 58.3% in patients with Stage III or IV cancer. On the contrary, Sajisevi 11, Morand 12, and Hong 25 found 22%, 28.5%, and 21% positive margins, respectively, when analysing low-intermediate-staged tumours. Achieving negative margins in advanced tumours may be difficult and, in some cases, extending the resection to adjacent structures according to the direction of infiltration may not be enough to obtain negative margins 26.
Timely adjuvant radiotherapy delivered in the presence of previously mentioned high-risk clinicopathological features such as positive margins has documented survival benefits 24,25. The recognition of these adverse prognostic factors suggests a role for intensifying treatment in this group of patients. Adjuvant radiotherapy is widely suggested to improve loco-regional control and survival in the presence of positive surgical margins 20,27. Nevertheless, despite bimodal therapy for aggressive disease, loco-regional failure, distant metastases, and poor survival have been frequently reported in presence of certain prognostic features 16.
For low- and intermediate-grade salivary carcinomas with negative margins and without high-risk features, evidence suggests that additional treatment is not necessary 11. Sajisevi 11 considered a study sample of 865 patients with low- to intermediate-grade MSGM and found that close (≤ 1 mm) and wider surgical margins (> 1 mm) had similar local control rates, and that adjuvant radiation for close margins was not associated with poorer local recurrence rates. He concluded that postoperative radiotherapy for positive margins was associated with a decreased risk of local recurrence compared to patients who did not undergo radiation. However, in isolation from other possible risk factors for local recurrence, selected patients with close surgical margins (≤ 1 mm) may be considered for observation. These conclusions are shared by other authors 12 who have shown that postoperative radiation may reduce loco-regional recurrence in some low- and intermediate-grade salivary gland cancers with adverse features, whereas it had no benefit in patients affected by early-stage and low-grade salivary gland cancer with both negative and close margins. Lee 28 considered 1,784 patients with non-metastatic AdCC of the parotid and observed that for positive margins the 5-year OS was 62.4% for surgery alone compared to 79.5% for adjuvant radiotherapy (p = 0.001), concluding that OS after bimodal treatment improved in patients with positive margins.
Surgical resection is not typically considered for cT4b tumours due to the difficulty in safely achieving negative margins. Patel 24, however, described a large series of 974 patients with cT4b major salivary gland malignancy, a minority of which (22.4%) underwent definitive treatment either with surgical resection and adjuvant therapy (67.6%), radiotherapy alone or chemoradiotherapy. He registered a high rate of positive surgical margins (65.4%) in patients undergoing surgical resection and adjuvant treatment, unsurprisingly, considering the stage of tumours. Patel concluded that surgical resection and adjuvant therapy is the most effective treatment for management of advanced-stage MSGM, but negative margins are not mandatory, since they do not significantly affect OS. Analogously, Stodulski 14 analysed a series of 40 patients with parotid gland SDC, a clinically aggressive cancer with high risk of local recurrence and distant metastases, and described no significant difference in OS based on margin status (p = 0.872). These studies suggest that patients with advanced-staged disease or aggressive tumour biology have a poor prognosis even with aggressive treatment regardless of the resection margins. Rajasekaran 29, who analysed 4,431 patients with MEC of the parotid gland, on the contrary observed that positive surgical margins negatively impacted survival for advanced-staged tumours. Analogously, Schrank 30 reported that positive surgical margins were predictors of survival in a study on 247 patients with LCUC, a rare tumour with poor prognosis. Thus, further studies should clarify the predictive role of margin status in advanced-staged tumours, at least for the more common histological types.
Ali 9 showed that positive margins is a pathologic predictor of distant metastasis reporting that the 5-year distant recurrence-free probability was 88.8% for negative and 63.2% for positive margins (p < 0.0001) in a series of 301 cases. In contrast, Tam 17 found no significant association between distant metastasis and margin status, although this information was not available or unclear in 5% of the 200 patients considered.
There is general agreement regarding higher loco-regional recurrence and worse OS in patients with positive margins regardless of the histological type and the need to perform surgical resection achieving negative margins when feasible. This association was present despite adjustment for the use of postoperative radiotherapy 12,28. In contrast with these authors, Hu 13 analysed 60 patients who underwent parotidectomy and adjuvant radiotherapy for metastatic cutaneous SCC, finding a non-significant trend towards higher loco-regional failure for close and positive margins. In the same way, Wang 31, reviewing the medical records of 19 paediatric patients with ACC, did not find a significant relationship between margin status and OS or DFS. The heterogeneity of MSGMs and these data suggest the need for specific studies with larger series regarding the role of margins according to specific histological subtypes.
There is no agreement regarding the definition of what constitutes a close margin since the effective cutoff varies between studies. In 4 papers of this review which considered close margins 11,12,15,16, however, this definition included cancer at less than 1 mm from the cut tissue edge. Such a narrow definition of margin and the high rate of close or incomplete excision after major salivary gland removal is probably due to the anatomical features and the tendency of these neoplasms to infiltrate deeply 13. Association between close margins and increased distant, local recurrences, and lower OS was not unequivocally ascertained but it is generally considered that the close surgical margin itself is not a risk factor for recurrence in MSGM if surgery can obtain negative margins. Thus, partial removal of the affected gland can be justified with complete removal of the tumour in the gland 13,32. However, further and more accurate studies on the role and definition of close margins are warranted.
Given the frequency of close margins after surgery for a salivary gland tumour, the issue of whether or not to apply postoperative radiation is essential, especially given the associated morbidity of adjuvant treatments. Some single-institution analyses have suggested that irradiating salivary cancer without high-risk features for close surgical margins was not associated with improved oncologic outcomes 17,19. Therefore, in these cases, adjuvant radiotherapy is generally considered unnecessary.
All studies considered in this review are retrospective and include a heterogeneous group of patients with different MSGM subtypes which make it difficult to compare the data of individual studies in detail. MSGM are rare tumours of diverse morphology and more than 20 different histological subtypes have been identified. Due to the rare occurrence and diverse histologic features of salivary gland cancers, prospective studies are difficult to conduct. Furthermore, potentially significant differences between histological subtypes could be weakened, especially for the rarer histotypes. Retrospective studies are an important tool in the study of uncommon diseases, manifestations and outcomes, although they have several limitations due to their design. They depend on the review of documents and reports that were generally not designed to collect data for specific research, and some information or clinical variables potentially affecting results may be not available for every patient.
Conclusions
Most studies agree that achieving negative resection margins significantly reduces rates of local recurrence and improves long-term survival. In case of adverse clinical features, such as positive margins, adjuvant radiotherapy is strictly recommended. The definition of close margins varies widely among studies and a correlation between close margin and oncological outcomes is not highlighted. The watch-and-wait approach with intensive follow-up is generally considered justified in case of a close margin after surgical excision.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
MdV, GP, VM: study design, article selection, review drafting, writing, and critical revision; VDS, EC: article selection, data extraction, critical revision; DA, FZ: article search and selection; CP, GS, AG: critical revision of the article.
Ethical consideration
Not applicable.
History
Received: March 6, 2025
Accepted: March 18, 2025
Figures and tables
Figure 1. PRISMA flow diagram of the review.
| First author | Year | Enrollment period | No. of patients | Brief summary | Gland(s) involved | Histological types | Positive margins | Close margin | Impact of margin status on OS and/or DFS and/or LRR |
|---|---|---|---|---|---|---|---|---|---|
| Kucharska33 | 2024 | 2010-2020 | 63 | Age over 65 years is a significant factor associated with higher risk of disease recurrence and impaired survival outcomes | Parotid: 50 (79.4%)Submandibular: 13 (20.6%) | AdCC: 17 (27%)AC: 11 (17.4%)MEC: 9 (14.3%)ACC: 9 (14.3%)SCC: 9 (14.3%)Other: 8 (12.7%) | 9 (14.3%) | No data | No data |
| Patel24 | 2024 | 2004-2019 | 976 (148 surgery + RT) | Patients with cT4b salivary cancer with positive surgical margins had higher 5-year OS than those undergoing definitive (C)RT (48.5% vs 30.1%, p = 0.018) and similar 5-year OS as those with negative margins (48.5% vs 54%, p = 0.205) | Parotid: 135 (91.2%)Submandibular: 4 (2.7%)Unspecified: 9 (6.1%) | AdCC: 22 (14.9%)AC: 14 (9.5%)MEC: 18 (12.2%)SCC: 30 (20.3%)Other: 64 (43.2%) | 89 (65.4%) | No data | Any impact of positive margins on survival in cT4b |
| Sajisevi11 | 2024 | 2010-2019 | 865 | In low- to intermediate-grade parotid and submandibular carcinomas: (1) close (≤ 1 mm) and wider (> 1 mm) surgical margins have similar local control; (2) adjuvant RT for close margins was not associated with local recurrence rates. Selected patients with low- to intermediate-grade cancers with close surgical margins may be safely considered for observation | Parotid: 801 (93%)Submandibular: 64 (7%) | AdCC: 239 (28%)AC: 41 (5%)MEC: 424 (49%)SCC: 74 (9%)Other: 87 (10%) | 192 (22%) | Close margins (≤ 1 mm): 395 (59%) | Increase in risk of local recurrence with positive margins (R1/R2 vs R0: HR, 2.47; 95% CI, 1.26-4.82). |
| Morand12 | 2023 | 2010-2020 | 621 | Postoperative RT may reduce loco-regional recurrence in some low- and intermediate-grade salivary gland cancers with adverse features such as positive margins, but it had no benefit in patients with early-stage, low-grade salivary gland cancer and negative margins | Parotid: 588 (94.7%)Submandibular: 33 (5.3%) | AdCC: 182 (29.3%)MEC: 312 (50.2%)Other: 127 (20.5%) | 177 (28.5%) | Close margins (< 1 mm): 140 (22.5%) | Close/positive vs negative marginsLoco-regional recurrence: p = 0.01Disease-specific death: p = 0.02 |
| Coleman22 | 2023 | 2004-2018 | 967 | Major salivary gland malignancies in paediatric patients exhibit variations in histopathologic characteristics by age, gender, and race. Negative margins impact 5-year survival rates, especially in high-stage tumours | Parotid: 833 (86%)Submandibular: 98 (10%)Unspecified: 36 (4%) | AdCC: 72 (7.4%)AC: 32 (3.3%)ACC: 325 (33.6%)MEC: 399 (41.3%)Other: 139 (14.4%) | 241 (24.9%) | No data | 5-year OS 94.9%Negative margins showed a survival rate of 94%Positive margins had a survival rate of 80%p = 0.003 |
| Hu13 | 2023 | 2008-2018 | 60 | Adjuvant RT is an established component in the management of metastatic cutaneous SCC involving the parotid gland. High loco-regional control rates were associated with the routine use of such an adjuvant treatment | Parotid: 60 (100%) | SCC: 60 (100%) | 20 (33.3%) | 30 (50%)Defined as margin < 2 mm | 2- and 5-year OS was 76% and 60%Surgical margins were not significantly associated with loco-regional failure |
| Talwar34 | 2023 | 2004-2018 | 154 (134 surgery) | CS is a rare salivary gland tumour frequently diagnosed at locally advanced stages. Despite multimodal treatment, outcomes remain poor. In multivariable analysis, advanced pT category, pN1, and positive margins are associated with worse survival | Parotid: 122 (86%)Submandibular: 21 (14%)Unspecified: 11 (7%) | CS: 154 (100%) | 43 (32%) | No data | 3-year OS was 57.6%3-year survival rates were 69.6% (95% CI, 58.7%-82.4%) for patients with negative margins and 46.1% (95% CI, 31.9%-66.8%) for patients with positive margins |
| Hong25 | 2022 | 2007-2017 | 655 | In early-stage major salivary gland cancers, patients with positive margins had a higher loco-regional recurrence and those with moderate/poor differentiation had a worse DSS. Stratified analysis indicated no protective effects from the use of adjuvant RT | Parotid: 479 (73%)Submandibular: 134 (20%)Sublingual: 29 (4%)Unspecified: 11 (2%) | AdCC: 148 (22.6%)AC: 34 (5.2%)ACC: 136 (20.8%)MEC: 175 (26.8%)Other: 162 (24.7%) | 138 (21%) | No data | Patients with positive margins had a higher loco-regional recurrence (aHR, 3.48; 95% CI, 1.62-7.48) and those with moderate/poor differentiation had a worse DSS (aHR, 1.34; 95% CI, 1.08-1.65; aHR, 1.61; 95% CI, 1.06-2.44) |
| Sideris20 | 2021 | 1979-2018 | 32 | DFS and OS in AdCC of the salivary glands is excellent with surgery as the first-line treatment. Poor survival outcomes are uncommon and may be associated with locally-advanced disease in the presence of other well-established high-risk features | Parotid: 30 (93.8%)Submandibular: 1 (3.1%)Sublingual: 1 (3.1%) | AdCC: 32 (100%) | 18 (56.3%) | No data | Positive margins were associated with recurrence:log-rank test (Mantel-Cox) positive margins p = 0.03 |
| Lukovic35 | 2020 | 2000-2017 | 1,035 | Development and validation of a prediction score for distant metastasis in MSGM | Parotid: 847 (82%)Submandibular: 188 (18%) | AdCC: 195 (19%)AC: 75 (7%)ACC: 237 (23%)MEC: 207 (20%)CEPA: 68 (7%)SDC: 116 (11%)Other: 137 (13%) | 373 (37%) | No data | Positive margins were associated with recurrenceunivariable and multivariable analysis: p < 0.01 |
| Terada36 | 2020 | 2004-2015 | 60 | Lymph node density (LND) is a predictor of outcomes for major salivary gland carcinoma without clinical lymph nodes metastasis. An LND ≥ 0.1 was significantly associated with a short OS (p < 0.05). Multivariate analysis with adjustment for pN classification and positive surgical margins showed that an LND ≥ 0.1 is a predictor of OS | Parotid: 40 (66.7%)Submandibular: 14 (23.3%)Sublingual: 6 (10%) | AdCC: 17 (28.3%)AC: 6 (10%)ACC: 5 (8.3%)MEC: 10 (16.7%)CEPA: 13 (21.7%)SDC: 5 (8.3%)Other: 4 (6.7%) | 14 (23.3%) | No data | Positive margins were associated with recurrence:univariable and multivariable analysis: present/absent HR 5.63 (1.94–16.30) p<0.01 |
| Wang31 | 2019 | 1998-2015 | 19 | Analysis of the role of surgery in the treatment of ACC of the major salivary gland in pediatric patients | Parotid: 17 (89.5%)Submandibular: 1 (5.3%)Sublingual: 1 (5.3%) | ACC: 19 (100%) | 5 (26.3%) | No data | Surgical margins were not significantly associated with OS and DFS |
| Stodulski14 | 2019 | 1996-2015 | 40 | Assessment of treatment results of parotid gland SDC | Parotid: 40 (100%) | SDC: 40 (100%) | 16 (40%) | 6 (15%)from 1 to 3 mm | Any impact of positive margins on survival (p = 0.872) |
| Cheraghlou37 | 2018 | 2004-2016 | 1,015 | RT may improve survival in cases with at least one high-risk adverse feature: high grade, positive surgical margins, and, for salivary SCC, positive extracapsular extension | Parotid: 849 (83.6%)Submandibular: 131 (12.9%)Sublingual: 8 (0.8%)Unspecified: 27 (2.7%) | SCC: 368 (36.3%)MEC: 174 (17.1%)ACC: 79 (7.8%)AdCC: 111 (10.9%)AC: 200 (19.7%)Other: 83 (8.2%) | 462 (45.5%) | No data | Multivariate analysis of factors associated with survival:SCCHR Positive 1.350Non-SCCHR Positive 1.093 |
| Rajasekaran29 | 2018 | 2004-2012 | 4,431 | Factors associated with decreased survival were advanced age, comorbidities, high tumour grade, high stage, and positive surgical margins. Female gender was the only factor associated with improved survival. | Parotid: 4,431 (100%) | MEC: 4,431 (100%) | 1,093 (24.7%) | No data | Positive margins were associated with worse OS5-year survivalPositive 66.8%5-year survivalNegative 82.9% |
| Lee28 | 2017 | 2004-2012 | 1,784 | Analysis of practice patterns and outcomes of postoperative RT for AdCC | Parotid: 923 (51.7%)Submandibular: 693 (38.8%)Other: 168 (9.4%) | AdCC: 1,784 (100%) | 817 (45.8%)No RT 166 (35.2%)RT 651 (49.6%) | No data | Positive margins were associated with worse OSUnivariableHR Positive 1.49MultivariableHR Positive 1.24 |
| Schrank30 | 2017 | 1998-2012 | 247 | Major salivary gland LCUC is rare and has a poor prognosis. Characterisation of patient demographics, tumour characteristics, and predictors of outcome have been limited by low case numbers, as well as grouped analysis with other salivary malignancies. The objective of this study was to address these issues using large-scale national data | Parotid: 221 (89.5%)Submandibular: 25 (10.1%)Sublingual: 1 (0.4%) | LCUC: 247 (100%) | 77 (43%) | No data | Positive margins were associated with worse OSPositive margins = 31%Negative margins = 48% |
| Amini38 | 2016 | 1998-2016 | 2,210 | In an analysis of data from the National Cancer Data Base on 2,210 patients with salivary gland carcinomas, OS was significantly inferior with adjuvant CRT compared with RT alone on multivariate analysis | Parotid: 1,852 (83.8%)Submandibular: 276 (12.5%)Sublingual: 16 (0.7%)Unspecified: 66 (3%) | MEC: 1,032 (6.7%)AdCC: 145 (6.6%)AC: 843 (38.1%)SDC: 106 (4.8%)ACC: 84 (3.8%) | 1,157 (52.3%) | No data | Median OS for the entire cohort was 63.7 months5-year OS 52.1%Variables associated with inferior OS included positive margins |
| Sayan15 | 2016 | 2006-2015 | 28 | Comparison of clinical outcomes and toxicity profile among a retrospective cohort of patients with primary major salivary gland carcinomas treated with surgery followed by adjuvant RT vs. surgery and adjuvant CRT | Parotid: 25 (89.3%)Submandibular: 3 (10.7%) | MEC: 13 (46.4%)AdCC: 6 (21.4%)Other: 9 (32.1%) | 15 (53.6%) | 4 (14.3%)Defined as margin < 0.5 mm | 3-year OS was 100% with S+RT and 87.5% with S+CRT (p = 0.141)No data regarding margin status and OS, DFS or LRR |
| Thompson39 | 2016 | 1990-2015 | 25 | ACC with high-grade transformation is a rare variant composed of both conventional low-grade ACC and a separate high-grade component. The clinicopathologic and immunohistochemical features of 25 cases are herein described | Parotid: 25 (100%) | ACC: 25 (100%) | 13 (52%) | No data | No data |
| Zhan40 | 2016 | 1998-2012 | 278 | OC has a poor long-term prognosis and lymph node metastases are common. Distant and regional metastases are significant predictors of decreased survival | Parotid: 246 (88.5%)Submandibular: 32 (11.5%) | OC: 278 (100%) | 123 (44.2%) | No data | 5-year OSM+:55%M-:74%10-year OSM+:27%M-:49%Positive margins were associated with worse OS |
| Hosni16 | 2015 | 2000-2012 | 304 | Report of outcomes of postoperative RT for major salivary gland carcinoma and identification of patients at high risk for distant metastases | Parotid: 237 (78%)Submandibular: 63 (21%)Sublingual: 4 (1%) | MEC: 56 (18%)AdCC: 55 (18%)ACC: 49 (16%)SDC: 40 (13%)Other: 104 (35%) | 152 (50%) | Very close (< 1 mm) 98 (32%)Close (< 5 mm) 22 (7%) | Positive margins predicted distant metastases and cause-specific survival |
| Ali9 | 2015 | 1985-2009 | 301 | Report of the incidence of distant metastases in salivary gland cancer as well as the histotypes most commonly associated with it, identifying factors predictive of such an occurrence | Parotid: 266 (88%)Submandibular: 30 (10%)Sublingual: 5 (2%) | SDC: 17 (5.6%)AC: 33 (11%)SCC: 30 (10%)CEPA: 59 (19.6%)ACC: 37 (12.3%)AdCC: 28 (9.3%)MEC: 94 (31.2%)Other: 3 (1%) | Close/positive145 (48.2%) | Not defined | Distant metastasis in M0 respect to margin status:Negative 125 (88.8%)Close/positive 145 (63.2%)p < 0.0001 |
| Katabi21 | 2014 | 1984-2009 | 52 | Comparison of different grading systems of MEC and identification of the most reliable and objective histopathologic features predictive of outcome | Parotid: 50 (96%)Submandibular: 2 (4%) | MEC: 52 (100%) | Close/positive margins 19 (36.5%) | Not defined | Margin status was associated with recurrence-free survival |
| Ali10 | 2014 | 1985-2009 | 301 | Proposal of a nomogram predictive of survival in salivary gland cancer | Parotid: 266 (88%)Submandibular: 30 (10%)Sublingual: 5 (2%) | SDC: 17 (5.6%)AC: 33 (11%)SCC: 30 (10%)CEPA: 13 (4.3%)ACC: 37 (12.3%)AdCC: 28 (9.3%)MEC: 94 (31.2%)Other: 3 (1%) | Close/positive 158 (52.5%) | Not defined | 5-year survivalpositive/close158 (62%)Negative113 (80.6%)p = 0.043 |
| Tam17 | 2013 | 1990-2011 | 200 | Excellent loco-regional control can be achieved in major salivary gland tumours treated with surgery and postoperative RT. The cumulative incidence and predictors of distant metastasis in high-risk major salivary gland tumours were analysed | Parotid: 167 (84%)Submandibular: 32 (16%)Sublingual: 1 (0.5%) | SDC: 18 (9%)AC: 45 (23%)ACC: 29 (15%)AdCC: 31 (16%)MEC: 52 (25%)Other: 25 (18%) | 93 (47%) | 53 (27%)Not defined | Distant metastasis compared to margin status:Negative 141 (21%)Close 53 (27%)Positive 193 (47%)p = 0.72 |
| Kim41 | 2012 | 1998-2010 | 35 | Clinical outcomes and prognostic factors in 35 patients with SDC treated postoperatively with adjuvant RT were described | Parotid: 22 (62.9%)Submandibular: 12 (34.3%)Sublingual: 1 (2.8%) | SDC: 35 (100%) | 17 (48.6%) | No data | Multivariate analysis showed that margin status is not an unfavorable prognostic factor |
| McHugh18 | 2012 | 1990-2007 | 125 | Analysis of the impact of clinical and pathologic findings on disease outcomes in MEC. High histological grade, advanced stage, perineural invasion, positive surgical margins, and submandibular location all portend for poor outcomes | Parotid: 108 (86.4%)Submandibular: 15 (12%)Sublingual: 2 (1.6%) | MEC: 125 (100%) | 51 (46.9%) | Close 26 (24.3%)Not defined | Positive vs negativeOS 73.3% vs 90.9% p = 0.09DFS 68% vs 89.1% p = 0.04 |
| Tanvetyanon19 | 2009 | 1998-2007 | 24 | Analysis of the potential value of postoperative CRT in patients with high-risk salivary gland carcinomas | Parotid: 20 (83.3%)Submandibular: 3 (12.5%)Sublingual:1 (4.2%) | SDC: 5 (20.8%)AdCC: 2 (8.3%)MEC: 11 (45.8%)CEPA: 4 (16.7%)Undifferentiated carcinoma: 2 (8.3%) | 14 (58.3%) | 6 (25%)Not defined | No data |
| Chen27 | 2007 | 1960-2004 | 207 | Lymph node metastasis, high tumour grade, positive margins, and T3-4 categories predict significant rates of LRR after surgery for carcinomas of the major salivary glands. Postoperative RT should be considered for patients with these disease characteristics | Parotid: 135 (65%)Submandibular: 60 (29%)Sublingual:12 (6%) | AC: 16 (8%)SCC: 30 (10%)OC: 6 (3%)ACC: 34 (16%)AdCC: 50 (24%)MEC: 67 (32%)Other: 4 (1%) | 60 (30%) | No data | 10-year loco-regional control by margin status (No. of failures/margin status)Negative: 24/147Positive: 18/60p = 0.01 |
| OS: overall survival; DFS: disease free survival; LRR: loco-regional recurrence; DM: distant metastasis; MEC: mucoepidermoid carcinoma; AdCC: adenoid cystic carcinoma; AC: adenocarcinoma; ACC: acinic cell carcinoma; SCC: squamous cell carcinoma; SDC: salivary duct carcinoma; OC: oncocytic carcinoma; LCUC: large cell undifferentiated carcinoma; CS: carcinosarcoma; CEPA: carcinoma ex-pleomorphic adenoma. | |||||||||
References
- Guzzo M, Locati L, Prott F. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. 2010;74:134-148. doi:https://doi.org/10.1016/j.critrevonc.2009.10.004
- Swid M, Li L, Drahnak E. Update salivary gland immunohistochemistry: a review. Arch Pathol Lab Med. 2023;147:1383-1389. doi:https://doi.org/10.5858/arpa.2022-0461-RA
- Sood S, McGurk M, Vaz F. Management of salivary gland tumors: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149. doi:https://doi.org/10.1017/S0022215116000566
- Lee R, Tan A, Tong E. Epidemiology, prognostic factors, and treatment of malignant submandibular gland tumors: a population-based cohort analysis. JAMA Otolaryngol Head Neck Surg. 2015;141:905-912. doi:https://doi.org/10.1001/jamaoto.2015.1745
- NCCN clinical practice guidelines in oncology. Head and Neck Cancers Version 2.2018.
- Lombardi D, Tomasoni M, Lorini L. Baseline prognostic factors affecting survival in recurrent and/or metastatic salivary gland adenoid cystic carcinoma. Oral Oncol. 2022;126. doi:https://doi.org/10.1016/j.oraloncology.2022.105764
- Benchetrit L, Morse E, Judson B. Positive surgical margins in submandibular malignancies: facility and practice variation. Otolaryngol Head Neck Surg. 2019;161:620-628. doi:https://doi.org/10.1177/0194599819852094
- McInnes M, Moher D, Thombs B. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies. JAMA. 2018;319:388-396. doi:https://doi.org/10.1001/jama.2017.19163
- Ali S, Bryant R, Palmer F. Distant metastases in patients with carcinoma of the major salivary glands. Ann Surg Oncol. 2015;22:4014-4019. doi:https://doi.org/10.1245/s10434-015-4454-y
- Ali S, Palmer F, Yu C. Postoperative nomograms predictive of survival after surgical management of malignant tumors of the major salivary glands. Ann Surg Oncol. 2014;21:637-642. doi:https://doi.org/10.1245/s10434-013-3321-y
- Sajisevi M, Nguyen K, Callas P. Oncologic safety of close margins in patients with low- to intermediate-grade major salivary gland carcinoma. JAMA Otolaryngol Head Neck Surg. 2024;150:107-116. doi:https://doi.org/10.1001/jamaoto.2023.3952
- Morand G, Eskander A, Fu R. The protective role of postoperative radiation therapy in low and intermediate grade major salivary gland malignancies: a study of the Canadian Head and Neck Collaborative Research Initiative. Cancer. 2023;129:3263-3274. doi:https://doi.org/10.1002/cncr.34932
- Hu M, Kim A, Emeto T. Metastatic cutaneous squamous cell carcinoma to the parotid: adjuvant radiotherapy and treatment outcomes. J Med Radiat Sci. 2023;70:161-170. doi:https://doi.org/10.1002/jmrs.650
- Stodulski D, Mikaszewski B, Majewska H. Parotid salivary duct carcinoma: a single institution’s 20-year experience. Eur Arch Otorhinolaryngol. 2019;276:2031-2038. doi:https://doi.org/10.1007/s00405-019-05454-0
- Sayan M, Vempati P, Miles B. Adjuvant therapy for salivary gland carcinomas. Anticancer Res. 2016;36:4165-4170.
- Hosni A, Huang S, Goldstein D. Outcomes and prognostic factors for major salivary gland carcinoma following postoperative radiotherapy. Oral Oncol. 2016;54:75-80. doi:https://doi.org/10.1016/j.oraloncology.2015.11.023
- Tam M, Riaz N, Salgado L. Distant metastasis is a critical mode of failure for patients with localized major salivary gland tumors treated with surgery and radiation. J Radiat Oncol. 2013;2:285-291. doi:https://doi.org/10.1007/s13566-013-0107-6
- McHugh C, Roberts D, El-Naggar A. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer. 2012;118:3928-3936. doi:https://doi.org/10.1002/cncr.26697
- Tanvetyanon T, Qin D, Padhya T. Outcomes of postoperative concurrent chemoradiotherapy for locally advanced major salivary gland carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:687-692. doi:https://doi.org/10.1001/archoto.2009.70
- Sideris A, Rao A, Maher N. Acinic cell carcinoma of the salivary gland in the adult and paediatric population: a survival analysis. ANZ J Surg. 2021;91:1233-1239. doi:https://doi.org/10.1111/ans.16421
- Katabi N, Ghossein R, Ali S. Prognostic features in mucoepidermoid carcinoma of major salivary glands with emphasis on tumor histologic grading. Histopathology. 2014;65:793-804. doi:https://doi.org/10.1111/his.12488
- Coleman M, Liang J, Rastatter J. Exploring the epidemiology and survival trends in pediatric major salivary gland malignancies: insights from the National Cancer Database. Curr Oncol. 2023;30:6134-6147. doi:https://doi.org/10.3390/curroncol30070456
- Geiger J, Ismaila N, Beadle B. Management of salivary gland malignancy: ASCO Guideline. J Clin Oncol. 2021;39:1909-1941. doi:https://doi.org/10.1200/JCO.21.00449
- Patel A, Haleem A, Choudhry H. Surgical resection improves overall survival in cT4b major salivary gland cancer. Otolaryngol Head Neck Surg. 2024;170:1349-1363. doi:https://doi.org/10.1002/ohn.686
- Hong W, Chang S, Tsai C. The effect of adjuvant radiotherapy on clinical outcomes in early major salivary gland cancer. Head Neck. 2022;44:2865-2874. doi:https://doi.org/10.1002/hed.27203
- de Vincentiis M, Magliulo G, Soldo P. Extended parotidectomy. Acta Otorhinolaryngol Ital. 2005;25:169-173.
- Chen A, Granchi P, Garcia J. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 2007;67:982-987. doi:https://doi.org/10.1016/j.ijrobp.2006.10.043
- Lee A, Givi B, Osborn V. Patterns of care and survival of adjuvant radiation for major salivary adenoid cystic carcinoma. Laryngoscope. 2017;127:2057-2062. doi:https://doi.org/10.1002/lary.26516
- Rajasekaran K, Stubbs V, Chen J. Mucoepidermoid carcinoma of the parotid gland: a National Cancer Database study. Am J Otolaryngol. 2018;39:321-326. doi:https://doi.org/10.1016/j.amjoto.2018.03.022
- Schrank T, Zhan K, Lentsch E. Predictors of outcomes in large cell undifferentiated carcinoma of the major salivary glands. Laryngoscope. 2017;127:372-376. doi:https://doi.org/10.1002/lary.26136
- Wang C, Mao M, Li B. Surgery alone is effective in the management of pediatric salivary gland acinic cell carcinoma. J Oral Maxillofac Surg. 2019;77:1713-1723. doi:https://doi.org/10.1016/j.joms.2019.01.044
- Cho J, Lim B, Kim E. Low-grade salivary gland cancers: treatment outcomes, extent of surgery and indications for postoperative adjuvant radiation therapy. Ann Surg Oncol. 2016;23:4368-4375. doi:https://doi.org/10.1245/s10434-016-5353-6
- Kucharska E, Rzepakowska A, Żurek M. Oncologic outcomes of the most prevalent major salivary gland cancers: retrospective cohort study from single center. Eur Arch Otorhinolaryngol. 2024;281:4305-4313. doi:https://doi.org/10.1007/s00405-024-08650-9
- Talwar A, Patel E, Tam M. Patterns of care and outcomes of carcinosarcoma of the major salivary glands. Otolaryngol Head Neck Surg. 2023;168:775-781. doi:https://doi.org/10.1177/01945998221120646
- Lukovic J, Alfaraj F, Mierzwa M. Development and validation of a clinical prediction-score model for distant metastases in major salivary gland carcinoma. Ann Oncol. 2020;31:295-301. doi:https://doi.org/10.1016/j.annonc.2019.10.024
- Terada H, Suzuki H, Hanai N. Prognostic value of lymph node density for major salivary gland carcinoma without clinical lymph node metastasis. Am J Otolaryngol. 2020;41. doi:https://doi.org/10.1016/j.amjoto.2019.102304
- Cheraghlou S, Yu P, Otremba M. Extracapsular extension is not a significant prognostic indicator in non-squamous cancers of the major salivary glands. Cancers Head Neck. 2018;3. doi:https://doi.org/10.1186/s41199-018-0032-x
- Amini A, Waxweiler T, Brower J. Association of adjuvant chemoradiotherapy vs radiotherapy alone with survival in patients with resected major salivary gland carcinoma: data from the National Cancer Data Base. JAMA Otolaryngol Head Neck Surg. 2016;142:1100-1110. doi:https://doi.org/10.1001/jamaoto.2016.2168
- Thompson L, Aslam M, Stall J. Clinicopathologic and immunophenotypic characterization of 25 cases of acinic cell carcinoma with high-grade transformation. Head Neck Pathol. 2016;10:152-160. doi:https://doi.org/10.1007/s12105-015-0645-x
- Zhan K, Lentsch E. Oncocytic carcinoma of the major salivary glands: a population-based study of 278 cases. Head Neck. 2016;38:E1981-E1986. doi:https://doi.org/10.1002/hed.24363
- Kim J, Lee S, Cho K. Treatment results of post-operative radiotherapy in patients with salivary duct carcinoma of the major salivary glands. Br J Radiol. 2012;85:E947-E952. doi:https://doi.org/10.1259/bjr/21574486
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1069 times
- PDF downloaded - 299 times



