Head and neck
Vol. 45: 111TH CONGRESS SIOECHCF - OFFICIAL REPORT 2025
Margins in head and neck non-melanoma skin cancer surgery: clinical/pathological criteria and their impact on oncological outcomes and therapeutic choices. A systematic review
Abstract
Introduction. Non-melanoma skin cancers (NMSCs), including basal (BCC) and squamous cell carcinoma (SCC), are the most prevalent malignancies affecting the skin, with the head and neck region being the most common site of involvement. Surgical excision remains the primary treatment modality. The role of surgical margins in the treatment of skin SCC and BCC of the head and neck remains a subject of ongoing debate. Clear definitions and guidelines regarding adequate surgical margins, as well as their impact on recurrence rates and overall outcomes, are critical for improving clinical management. This systematic review aims to evaluate the current literature on the definitions of surgical margins for SCC and BCC of the head and neck, as well as their impact on local recurrence, disease free survival, and other patient-centred outcomes.
Materials and methods. We conducted a systematic review following the PRISMA guidelines. A comprehensive search was performed across multiple databases, including PubMed and Scopus, for studies published up to December 2024. Eligible studies included those that reported on surgical margin definitions, surgical outcomes, and recurrence rates for SCC and BCC of the skin in the head and neck region. Data were extracted and analysed for margin size and oncological outcomes.
Results. Following the application of inclusion and exclusion criteria, 30 studies have been retrieved for qualitative synthesis. Of these, 12 studies focused on SCC only, 14 on BCC only, and 4 on mixed histologies. Margin involvement rates ranged widely across the studies included (5-56%) as did thelocal recurrence rate (0-20%). This is associated with a variability of the surgical margin both for SCC and BCC, and of the definition of margin as close/negative at final pathology. Most studies do not define a threshold for close vs. negative margins at final pathology. All studies but one reported a significant correlation between positive margins and oncological outcomes, with particular regards to local recurrence.
Conclusions. The findings highlight a lack of consensus on the optimal surgical margins for SCC and BCC of the head and neck, suggesting that margins may need to be individualised based on tumour characteristics, location, and patient factors. In particular, the anatomical complexity of the head and neck region suggests to separately address different high-risk areas as nose/midface, periauricular, and periocular with specific recommendations also concerning clinical margins.
Introduction
Skin cancer is generally classified as melanoma or non-melanoma skin cancer (NMSC). Melanoma is the deadliest skin malignancy and is derived from mutated melanocytes, its biology and clinical behaviour are fairly specific, and luckily its incidence is much lower than that of NMSC.
Basal (BCC) and squamous cell carcinomas (SCC) are by far the most common forms of NMSC (respectively around 75% and 20% of all NMSC), as well as the most common malignancies in humans and are the histotypes on which we decided to focus the present systematic review. BCC is the most common form of skin cancer. An estimated 3.6 million cases of BCC are diagnosed in the United States each year 1. SCC is the second most common form of skin cancer. An estimated 1.8 million cases are diagnosed in the United States each year 2. Therefore, in the USA, the estimated prevalence rate of BCC is 525 per 100,000 persons, and that of SCC is 262 per 100,000 persons 2. At the global level, according to the Global Burden of Disease Cancer Collaboration 2017 data, there are 7.7 million incident cases of NMSCs, responsible for 65,000 deaths 3.
Both histotypes derive from epidermal keratinocytes for which UV radiation is the main mutagenic factor and for this reason the sun-exposed head and neck region is especially vulnerable, with 60-80% of NMSCs occurring in this area 4,5. Treatment of skin cancers of the head and neck is associated with additional issues in comparison with other areas in terms of cosmetic results, with a clear social impact, both because the head and neck area is the most visible in the daily life, and because the imperfections are in general more easily noticed 6. Notably the location in the head and neck area puts both SCC and BCC in the high (or very high) risk class according to most major international guidelines 7.
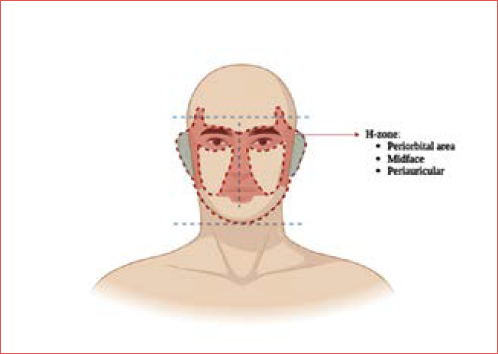
Within the head and neck skin surfaces, the H-zone (Cover figure) has been variously defined as a specific area on the face that is particularly vulnerable to the development of more aggressive BCC and SCC (for example SCC with a higher nodal metastatic risk). The issues of the H-zone are not specifically, or exclusively, related to a different biology of the lesions, rather than to the anatomic features of the areas involved. Some authors 8, in fact, prefer to refer to such high-risk areas in the face as periorificial areas (around eyelids, nostrils, mouth, and external auditory canal), because this position between internal surfaces and “external” skin and the pertinent anatomy is an issue by itself. Two clear examples, which in our opinion deserve a separate discussion, are skin malignancies arising in the midface around the nostrils, and those involving the external ear, where the diffusion, respectively along the nose tip cartilages and within the temporal bone, are the key factors influencing peculiar clinical behaviour.
Treatment options for NMSC include surgical excision, cryotherapy, chemotherapy, immunotherapy, and radiation. However, the standard practice is to surgically treat these lesions. Most NMSCs in Western Countries are managed by dermatologists and plastic surgeons 9, and prognosis is usually very good. However the high-risk, most problematic and advanced primary and recurrent cases are much more often referred to the head and neck surgeons.
Considering the extreme rarity of distant metastases, we can classify most of the reasons of referral to the head and neck surgeons in 2 categories: either neck nodal metastases (regional spread) or locally advanced, often recurrent, lesions (cT4a and some cT3 according to the AJCC/UICC classification) invading deep tissues and in particular bone (local spread). The second situation is most often due and/or leads to an incomplete or inadequate resection (positive or close margins). Therefore, when dealing with BCC and SCC, the issue of margins is particularly relevant for the otolaryngologist/head and neck surgeon and is the subject of the present systematic review.
The approach to the margins in NMSC impacts deeply on the surgical technique itself. In the “conventional” surgical resection the margins are fixed: the surgeon excises the tumour with a predefined distance of healthy tissue (e.g., 4, 5, or 10 mm), generally defined as clinical margin. By contrast, in surgery with Peripheral Deep En face Margin Assessment (PDEMA), and in particular in Mohs micrographic surgery, tissue is excised layer by layer, and margins are examined microscopically until no cancerous cells are evident at the margins. In Mohs surgery, the negative margin is therefore defined as the absence of tumour cells in a layer during the procedure itself.
Even if Mohs surgery has been designed and has become, according to many authors, a gold standard for treating certain types of skin cancers in cosmetically sensitive or functionally important areas, and specifically in the head and neck, it is actually contraindicated if a skin cancer has spread to deeper structures, or if the lesion is anyway so large to require reconstructive procedures, that is most often the case for those lesions referred to the otolaryngologist or head and neck surgeons 10-12. Furthermore, in case of Mohs surgery the margins are by definition negative, even if with different criteria than in conventional surgical excision, so that it is not possible to assess the impact of the variable “margins” itself. For these reasons, we are not considering PDEMA surgeries in the present systematic review.
In fact, we aim to assess which are the criteria to define a positive/close margin in case of “conventional” surgery, how the margins are assessed in the sample and how this impacts on oncological outcomes in general and on local relapse in particular.
Materials and methods
Protocol registration
The protocol of this systematic review and meta-analysis was registered on PROSPERO 13, an international database of prospectively registered systematic reviews in health and social care (Center for Reviews and Dissemination, University of York, York, UK), in January 2024 with registry number CRD42025631984.
Search strategy
A systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations 14. The electronic databases Scopus and PubMed were searched from database inception to 6th December 2024.
The inclusion criteria for selection of studies were chosen according to the PICOS tool: Patients (P), adults affected by NMSCs; Intervention (I), surgical removal; Comparator (C), none; Outcomes (O), overall survival (OS) (primary outcome), local control (LC), disease free survival (DFS), disease specific survival (DSS) (secondary outcomes); Study design (S), retrospective and prospective cohort studies.
The following Boolean search string have been used: ((NMSC) OR (non-melanoma skin cancer) OR (BCC) OR (SCC)) AND ((surgery) OR (resection) OR (excision)) AND (margin). The reference lists of all the included articles were thoroughly screened to find other relevant studies. References were exported to Zotero bibliography manager (v6.0.10, Center for History and New Media, George Mason University, Fairfax, VA, USA). After duplicate removal, 7 reviewers (SS, DM, AD, DC, AS, MB, and VM) independently screened all titles and abstracts and then evaluated the full texts of the eligible articles based on the inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus. In case of doubts, the full text was surveyed and, if a dispute remained, it was resolved by the 6 senior authors (FB, DR, GP, EZ, AG, and JG). No ethical approval nor informed consent were required for this study since all reported data were obtained from the available published literature.
Selection criteria
Studies were deemed eligible when the following inclusion criteria were met: (i) confirmed histopathological diagnosis of primary or recurrent NMSCs; (ii) patients treated with a surgical approach for the above-mentioned tumours; (iii) margin status clearly assessed; (iv) margin status and its correlation with at least one oncological outcomes; (v) studies reporting at least 5 cases.
Exclusion criteria were as follows: (i) inaccessibility to full text; (ii) overlapping cohorts or articles with data redundancy; (iii) lack of relevant clinicopathological data in terms of margin status and outcome data; (iv) series of Mohs micrographic surgery and other PDEMA surgeries; (v) mixed series where it is not possible to extrapolate numbers about head and neck cases; (vi) non-segregable data/inseparable cases between surgical and non-surgical cohorts; (vii) non-original studies (i.e. reviews, letters, editorials, or book chapters); (viii) animal model studies; (ix) non-English studies. The papers were thoroughly screened for duplicates.
Data extraction and quality assessment
Extracted data were collected in an electronic database including the first author, year of publication, country of origin, patients’ age and gender, period of treatment, type of surgery, type of multimodal treatment, staging system adopted, margin status and definition, follow-up, and outcomes.
The quality of the studies eligible for inclusion was categorised as poor, fair or good, in agreement with the National Institutes of Health quality assessment tool for Observational Cohorts and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed on 6th December 2024) 15. Seven reviewers (SS, DM, AD, DC, AS, MB, and VM) independently evaluated the papers, and any disagreement was resolved by discussion.
Results
Search results and risk of bias
The flow of studies through the review process is illustrated in the PRISMA diagram (Fig. 1). A total of 2,963 records were identified through database searching (PubMed and Scopus). After removing 1,038 duplicates and 171 records for other reasons (non-English language or studies on animals), 1,754 records were eligible for screening. After the first-stage screening, on the basis of title and abstract, 1,620 records were excluded and 134 papers were collected for full-text screening. Only one article was unavailable for retrieval. Following the application of inclusion and exclusion criteria, 101 studies were excluded, leaving 30 for qualitative synthesis. Of these, 12 focused on SCC only, 14 on BCC only, and 4 on mixed histologies.
Quality assessment
According to the National Institutes of Health Quality Assessment Tool for Observational Cohorts and Cross-Sectional Studies (NHLBI NIH Study Quality Assessment Tools) 15, 3 studies (10%) were deemed of good quality, 25 (83.3%) were fair, and 2 (6.7%) were poor due to a lack of clinical details.
Characteristics of the included studies
The included studies are summarised in Table I 16-45. A total of 6,196 patients were included in the studies. The sample size ranged from 21 to 1,062 patients. The included articles provided data on 14 different countries over a period of 19 years (2005-2024). Patient recruitment period ranged from 1980 to 2022. Patients’ age ranged from 3 to 101 years.
Most of the retrieved studies included all the head and neck area (n = 16, 53%), a few focused on the H-zone (n = 4, 13%), 2 on the lips, and only one on the nose and scalp, respectively. Notably, 6 studies evaluated the periauricular/external auditory canal region (20%).
Margin status was classified as negative, positive or close. Nine studies reported data on nodal involvement. Follow-up ranged from 1 to 279 months.
Margin involvement rates ranged widely across the studies included (5-56%) and so did local recurrence rate (0-20%). This is also associated with a variability of the clinical margin applied both for SCC and BCC, and of the definition of the margin as close/negative at final pathology. Most studies do not define a threshold for close vs. negative margin at final pathology.
Correlation between margin status and oncological outcomes was an inclusion criteria for retrieval, with most studies reporting direct correlation between positive margins and local recurrence. Only one study observed no correlation between positive margins and local recurrence 42. Data on margin status, local recurrence, and oncological outcomes are illustrated in Table II.
Discussion
In the present systematic review, we analyse the definition and subsequent impact of resection margins in case of surgical treatment for the first (BCC) and the second (SCC) most common malignancies in humans, when they arise in the head and neck area. Different considerations need of course to be drawn for the two different histotypes, which will be discussed separately in the following paragraphs.
The first outcome of the present review is the absence of a consensus about the safe surgical margin (clinical margin), reflected clearly in the absence of univocal recommendations in the main international guidelines, both for SCC and BCC 7, when it comes to high-risk lesions.
Not all the skin surface of the head and neck area has the same risk of developing aggressive and high-risk NMSC, which are more likely to be referred to head and neck surgeons rather than treated by dermatologists/plastic surgeons, possibly by Mohs surgery. In this regard, the so-called H-zone, essentially forming a region from the forehead down to the nose and extending outward to include the periorbital and periauricular areas, is of interest for three order of reasons:
- High risk of NMSC development: the frequent exposure to UV radiation in these areas, particularly the nose and surrounding regions, combined with the thinner and more delicate skin, predisposes individuals to skin damage and cancer formation.
- Issues in surgical resection and margin assessment: the complex anatomy of this area, including the adherence of the skin to the underlying cartilaginous and bony structures, often makes Mohs surgery not safe and not indicated as deep tissues are soon reached by cancer cells, and the same anatomy and ultrastructure of these drastically change the pattern of spread, independently from the histology and biology of such disease.
- Cosmetic and functional concerns: skin cancers in the H-zone can have significant cosmetic and functional implications. The proximity of the H-zone to vital structures, such as the eyes, nose, and mouth, means that treatment must be carefully planned to avoid scarring and preserve function. The impact on facial appearance can also affect patients’ self-esteem and quality of life, making early detection and treatment essential.
Functional concerns are particularly important when skin cancers involve the nose or periorbital area. Treatment of skin cancer in these regions requires not only complete excision but also careful reconstructive planning to ensure that both cosmetic and functional outcomes are optimised.
In particular, we do believe that, in a review aimed at otolaryngologists, within the H-zone, particular attention and specific consideration should be given to the perinasal/midface and to the periauricular/external ear areas, where the above mentioned issues are of particular concern and the general considerations about margins are challenged and lose at least in part validity especially due to the anatomical peculiarities and patterns of spread.
Basal cell carcinoma
BCC is a neoplasm with a low mortality, although these lesions may be locally aggressive and can recur after treatment, with a low metastatic rate between 0.003% and 0.01%. Apart from UV exposure, the other risk factors include advanced age, male gender, smoking, fair skin types I and II, arsenic exposure, and immunosuppression 46. The standard treatment of BCC is considered to be complete resection within clear margins.
Differently than SCC, BCC histopathologic subtypes are acknowledged to have a great impact on the risk of recurrence. BCCs with a low risk of recurrence include nodular, superficial, pigmented, infundibulo-cystic (BCC with adnexal differentiation), and fibroepithelial (Pinkus tumour). The subtypes with a high-risk of recurrence are the micronodular, infiltrating, sclerosing/morphoeic, basosquamous, and those with sarcomatoid differentiation 47. Other less frequently encountered variants are the keratotic, adenoid, clear cell, granular, and those with sebaceous/eccrine differentiation. In many cases, there may be also a combination of various morphological criteria 48.
The tendency to relapse, apart from the biological aggressiveness of the different histological variants, seems related (as for SCC) to the onset in critical areas, in particular the midface and periauricular region 34,49.
Other risk factors for recurrence include the patient’s age, non-primary tumours, cases treated before by Mohs micrographic surgery, those requiring multiple stages of Mohs surgery, BCC left for secondary healing after surgery, head and neck location, tumour depth (beyond fat), therapeutic modality adopted (curettage or photodynamic therapy having a higher risk of recurrence compared with excision and radiotherapy). The present review confirms the impact of margin involvement (both deep and superficial) on oncological outcomes 25,31,35. Low-risk situations for recurrence are young age and early stage, as well as reconstruction with a local skin flap 50,51.
There are several published guidelines on recommended clinical margins for BCC excisions, including 4 mm 26,27 and 3-5 mm 54,55 of uninvolved skin, and a depth of mid-subcutaneous adipose tissue. In NCCN guidelines, the recommended clinical margin is 4 mm for low-risk lesions, but it is considered “not feasible to recommend a defined margin for standard excision of high-risk BCC, due to the wide variability of clinical characteristics that may define a high-risk tumour” 7. Such inconsistency is reflected by the results of the present systematic review with the clinical margin for head and neck BCC ranging from 1 to 7 mm. More than two-thirds of the retrieved articles that report data regarding definition of surgical margins in BCC define a minimum value of 3 mm for a safe margin.
Furthermore, in the present review we can observe a clear variability in what the definition of a “close margin” among pathologists is. Many works do not define the threshold between what is defined as a negative and a close margin, and when they do, it ranges from around 0 (cancer cells at the edge) to 5 mm.
According to several authors 4,5,38 recurrent BCC and those larger than 2 cm should be managed with wider clinical margins than primary and smaller ones. Consistently, in primary BCC, the recurrence risk is 1.5%, while the rate increases to 13.2% when BCC has been previously treated 50.
Uhlman and colleagues 56 found that BCC lesions located in the head and neck anatomical region had a higher rate of incomplete excision (15%), compared to other anatomical regions. Specifically, anatomical regions of the head and neck such as the medial canthus, eyelid, and nose had high incomplete excision rates at 50%, 32%, and 23%, respectively. Tumour diameter ≥ 4 cm, head and neck location, and depth beyond fat are also associated with increased risks of death and metastasis 51.
Other risk factors for metastasis are male gender, history of prior radiation at the tumour site, and perineural invasion, although there is limited ability to predict metastatic BCC based on any of these features 51,57. Metastatic BCC spreading usually starts through the lymphatic system before invading the vascular supply 58.
Histopathological studies have shown that BCC with a tendency towards lymph node metastasis always show areas of squamous metaplasia, so that they are defined as basosquamous carcinomas, and must be considered as an intermediate form between BCC and SCC 59. In general, when a BCC gives nodal (or even distant) metastasis, the first reasonable attitude in our opinion is to question the original histological diagnosis and review the slides and sample, also with immunohistochemistry if needed.
Squamous cell carcinoma
Cutaneous SCC is a growing global health concern, particularly in the head and neck region, where it presents unique treatment challenges and a higher risk of regional metastasis, particularly to the neck nodes 32. Unlike BCC, SCC has a greater metastatic potential, making early and effective treatment critical. Risk factors apart from UV exposure, include history of prior skin cancer, immunosuppression (e.g., solid organ transplant recipients or chronic lymphocytic leukemia patients), male gender, and advanced age 24,32.
Determining adequate surgical margins remains a major controversy in skin SCC treatment, as there is no universal consensus on the optimal margin width. Guidelines vary, with recommendations ranging from 4 to 6 mm or more for high-risk tumours 32,39. Some authors propose 0.5 mm margins, while others recommend ≥ 6 mm, highlighting the need for evidence-based guidelines 24. This inconsistency leads to wide variations in clinical practice, particularly in anatomically constrained regions. Incomplete excision rates for head and neck skin SCC range from 7.6% to 15.9%, with deep margin involvement accounting for most cases 22, suggesting to prioritise deep margin clearance over lateral expansion. Emerging evidence, in fact, suggests that deep margins are more frequently involved than lateral ones, raising concerns about the effectiveness of wider peripheral excisions alone. Scalp SCC demonstrate particularly high deep margin failure rates 39. In a series of 114 patients with SCC of the scalp, 23 out of 114 (20%) experienced local recurrence 39. There was no significant difference in local recurrence rates between patients with clear excision margins and those with involved margins; in fact, the 5-year disease specific mortality rate was 20% in patients with clear excision margins compared to 35% in those with involved margins. The authors concluded that wider peripheral resection margins may not necessarily reduce the rate of incomplete excisions, as the deep margin is often the primary site of involvement.
Studies report that 60-80% of re-excisions for positive margins show no residual tumour, possibly due to an immune-mediated regression during wound healing. Also, among all the re-excision, 43% of positive deep margins contained residual tumour, compared to only 20% of cases with initial lateral margin involvement 24. Key risk factors for residual tumour include larger tumour size, greater Breslow thickness, high-risk anatomical locations (ear, nose, scalp), poor differentiation, and perineural invasion 24.
Many authors consider tumour thickness as one important parameter associated with biologic behaviour. Such measurement is currently performed in different ways depending on the anatomic location and subspecialty. Furthermore, the eighth edition of the AJCC staging system has changed the previously recommended method of measurement of skin SCC of the head and neck from a modified Breslow thickness to measuring from the granular layer of adjacent, normal-appearing skin to the deepest invasive tumour cell 60. In particular, Bovill et al. found that tumours ≥ 24 mm in diameter and 10.9 mm thick had a significantly higher risk of incomplete excision than smaller tumours 24. Moreover, the same authors stated that a delay between initial excision and re-excision has been linked to lower residual tumour rates, possibly due to an inflammatory response aiding tumour clearance 24. Amongst the different risk factors for recurrence, a statistically significant association was found between perineural involvement and subclinical spread (p = 0.012). According to Eskiizmir et al. perineural involvement is an uncommon feature of NMSCs, rarely detected clinically, but is associated with more aggressive tumours, larger tumour size, and a higher likelihood of recurrence 23.
Regarding the risk of regional metastasis in head and neck SCC of the skin, positive or close surgical margins (≤ 2 mm) significantly increase local and regional recurrences, with studies reporting regional recurrence rates of 29% in positive margin cases vs 5% in negative ones 24. Tumour thickness > 4 mm, lympho-vascular invasion, and perineural invasion further heighten the recurrence risk 27. Immunosuppression is another key factor, with organ transplant recipients and chronic lymphocytic leukemia patients showing higher metastatic rates 32. Some studies suggest that micrometastases around high-risk cutaneous SCC may explain recurrence even with clear surgical margins 24.
Lower lip and external ear, as well as scalp SCC, seem to have a relatively higher risk of developing local as well as parotid/cervical nodal metastases 39. In a series of patients with SCC of the lip, in fact, 33% of patients developed local recurrence despite clear margins, whereas 56% experienced recurrence with a close/positive margin. The majority of patients who relapsed after surgery, particularly within regional nodes, had a tumour thickness greater than 4 mm. The addition of local adjuvant radiotherapy in patients with a close/positive margin was associated with a significant improvement in recurrence free survival 27.
In another study with the aim to identify the risk of developing metastases to regional nodes in patients with cutaneous SCCs of the head and neck, the parotid gland represented the most common site of metastatic spread. Regional nodal metastasis following the excision of head and neck skin SCC was identified in 5.1% of cases, most of them within the first 2 years. The authors confirmed that lesions on the ear, poor differentiation, and incomplete excision margins were significant risk factors for developing regional lymph node metastasis 22. Interestingly, 60% of patients with metastases had cutaneous SCCs smaller than 20 mm in diameter. This finding aligns also with the study by Veness et al., which suggested that even small SCCs have metastatic potential 61.
Analysing the location of the lesion may help target lymph node basins for metastasis. Lesions on the cheek, pinna, temple, forehead, anterior scalp, and postauricular area tend to metastasise to the parotid basin and level II lymph nodes 62. Tumours from the posterior scalp are more likely to travel to level V, while midface SCCs (nose and lips) often bypass the parotid and involve levels I and, more rarely, also level III 63.
Nose and midface NMSC
NMSC of the nose presents a unique set of challenges, not only due to the intrinsic difficulties in treating cancer within this delicate and prominent anatomical region but also because of its anatomical complexity. In fact, the nose intricate structure of cartilages, including the lower lateral, upper lateral, and septum, creates natural reliefs and hollows that give the nose its characteristic shape, making the cosmetic restoration after ablative surgery extremely challenging. Furthermore, this complex tridimensional anatomy combined with the typical resistance of cartilage to tumour invasion may guide tumour growth towards sometimes unexpected and not straightforward paths, which must be taken into account when aiming at clear resection margins.
Diagnostic challenges: nasal vestibule malignancies
The cartilages are covered externally by skin and internally by mucosa making up for the nasal vestibule. Tumours arising from the nasal vestibule are mostly SCC, as those arising from the skin surface, and pose a relevant diagnostic challenge. In fact, travelling along the cartilaginous framework of the nasal valve (Fig. 2), they typically reach the nasal skin early, potentially invading the external surface of the nose before becoming evident in the nasal cavity, thus leading to a misdiagnosis of skin SCC. The involvement of internal and external layers of the nose tip implies that the standard surgical treatment of these lesions is rhinectomy. However, the misdiagnosis for NMSC often leads to a superficial resection or even to a Mohs surgery, which is not specific for the assessment of deep inter-cartilaginous spread, which can become negative at the following sections after the first detection. Ultimately such misdiagnosis most often leads to residual disease in the primary site, recurrence, and clinical and oncological disasters. Therefore we recommend that any skin lesion around the nostrils is checked endoscopically by an otolaryngologist to ensure that the malignancy has not originated from the nasal vestibule 6,64-67.
Reconstructive challenges in NMSC of the nose
The nose occupies a central position in the face, making it one of the most visible areas. Even small imperfections in the skin colour, texture, or shape after surgery can be immediately noticeable, leading to significant cosmetic concerns for the patient. Furthermore, as the skin of the nose, which is not only thin but also tightly adherent to the underlying cartilaginous framework, the cartilage can be involved or, more often, partially surrounded by the tumour and have to be removed, making reconstructive efforts much more difficult. In fact, once the cartilage is resected, it becomes virtually impossible to reconstruct this complex topography to the original form and this leads to cosmetic deformity and loss of support for the nasal skin itself. In addition, the same adherence of the skin and mucosa to the cartilage leads anyway to deformation and functional breathing issues as a consequence of the healing process.
The issue is compounded when a through resection is required, as this also raises the concern of reconstructing not only the outer skin but also the mucosal layer that lines the nasal cavity. Reconstructing the delicate mucosal lining of the nasal vestibule, which is integral to maintaining the function of the nose (such as airflow and protection against pathogens), is particularly challenging. Therefore, in bulky, high-risk NMSC, often referred to the otolaryngologist, when a wide surgical margin and a through resection are required, the difficulty of achieving functional and aesthetic reconstruction becomes a real challenge. In these cases, concerns about reconstruction should not but often negatively impact on oncological safety and therefore on the rate of complete resections/negative margins.
Risk of tumour spread and margin reliability
Furthermore, because the skin overlying the cartilages is so thin, resecting the tumour can also compromise the deep margin. This is particularly concerning when the surgical excision goes along the surface of the cartilage itself, as the thin skin may reduce the ability to maintain adequate margins, particularly in cases of SCC, where the risk of deep spread is greater. Also, the thinness of the skin may impact the depth of margin assessment, and achieving reliable margins may be more difficult than in other areas of the face where the skin is thicker.
An important consideration when dealing with NMSC of the nose is the resistance of cartilages to tumour spread. Cartilage generally serves as a barrier to the deep spread of tumour cells, which tend to slide along perichondrium. However, this can be an issue, as tumour cells will tend to infiltrate among the cartilages, leading to unexpected involvement of internal mucosal layer and potential recurrence. In these cases, the reliance on widening of margins based on the negativity of frozen sections can be risky, especially if the frozen sections analysis fails to detect microscopic tumour spread within the cartilage or along its edges. Knowledge of the anatomy of the nasal valve suggests that negative margins assessed on the tumour bed by frozen sections at the level of the edge of the cartilages, in particular between the alar and the lateral ones, cannot be relied upon, especially if the final assessment on the main surgical sample shows a deep involvement, and it should lead to a surgical revision with obvious oncological and reconstructive issues or to adjuvant radiotherapy. This appears of utmost importance because, as demonstrated in the present review, the achievement of clear surgical margins is generally agreed as the most important factor influencing local control, and adjuvant radiotherapy, at the dose required for an R1 resection, is associated to high short- and long-term toxicity rates affecting mostly nasal cosmesis and function 20,23,33.
The potential role of brachytherapy
Brachytherapy (also referred to in its modern form as interventional radiotherapy) involves bringing a radioactive source directly at the tumour site, delivering targeted radiation while sparing surrounding healthy tissue. Studies, such as those by Bussu et al., have highlighted the benefits of interventional radiotherapy in nasal vestibule SCCs 6,64,68-71, demonstrating excellent tumour control while avoiding the need for extensive surgical resection and complex reconstruction, and defining it as the standard therapeutic approach in these particular malignancies. Such technique has been employed and reported to be effective also for NMSC 72-74, and in our opinion should be considered in large, high-risk cases, when the above mentioned reconstructive challenges and difficulty to obtain clear margins become relevant issues.
Periauricular NMSC
Primary malignant tumours of the ear and temporal bone are rare, with dismal prognosis in advanced stages. Conversely, cutaneous cancers of the auricle and periauricular area spreading to the external ear and/or temporal bone are more frequent and warrant attention, since the deep margin of infiltration is often deeper than expected beyond the subcutaneous plane. Due to its rarity and specific features, no standardised classification systems or treatment guidelines exist 75. Periauricular and auricular cutaneous malignancies are challenging to manage, with higher recurrence rates and potentially worse prognosis than other head and neck skin cancers 26,28. The issue is complicated by the anatomical constraint of different tissues (skin, cartilage, nerves, bone, and dura mater) interacting with the tumour, in specific subsites, in a whole space of few millimetres. When the tumour extends medially beyond the skin, or is inadequately excised, it involves nerves, parotid gland, bony/cartilaginous structures, and easily the skull base. When it recurs deeply, it spreads laterally to the skin as the tip of an iceberg.
In our systematic review, 5-year OS ranged from 44.7% to 64.6%, while 5-year DSS varied from 62% to 77.6%. Surgical local control with clear margins was crucial for prognosis, with positive margins and invasive factors being key prognostic indicators 28,44. As a result, radical surgery remains the first-line treatment. Lateral temporal bone resection is widely accepted for primary temporal bone SCC at early stages or anteriorly located tumours in the periauricular area 76, while subtotal temporal bone resection has historically been considered highly complex and is indicated in advanced tumours with bone erosion. Recently, temporo-parotid resection has shown promising results, applying the principle of an en bloc resection of the parotid, adjacent skin together with the external auditory canal 77.
Margin status significantly impacts recurrence and DSS. Up to 50% of lesions with positive margins recur, and survival is notably worse in patients with involved margins 26,28,44,45,76. In advanced skin cancer extending medially, the goal is to achieve radical removal with clear margins, requiring to extend the resection to include the temporo-mandibular joint, facial nerve, or, less commonly, the infratemporal fossa. Despite being technically difficult, an en bloc specimen of the skin together with adjacent soft and bony tissues can be achieved 78,79. Although every effort should be warranted to preserve the facial nerve, clinical perineural invasion significantly affects survival and, according to the extent of disease and histology, nerve sacrifice may increase the possibility to achieve free margins. The quality of the resection obtained through an en bloc tumour specimen embedded within safe tissue seems also to provide a better quality of resection. Adjuvant radiotherapy is obviously mandatory in advanced case but does not preclude or reduce the value of achieving, or trying to achieve, free surgical margins, which remain the main prognostic aspect in skin cancer of this area 79.
Conclusions
The vast majority of studies agree that achieving clear resection margins significantly reduces local recurrence rates and improves long-term survival in both SCC and BCC of the head and neck. However, clinical margin recommendations vary widely across studies for both tumour types. In our view, beyond histopathological factors – particularly histological variants of BCC – the primary obstacle to consensus on clinical margins in head and neck NMSC lies in the distinct clinical behaviour of tumour based on their location. While the H-zone or periorificial areas are universally recognised as high-risk, they are often treated as a single entity in major guidelines, despite each site presenting unique clinical challenges driven more by anatomical complexity than tumour biology. Given that BCC and SCC are the most common malignancies in humans, the frequency of these tumours at different head and neck sites further justifies the need for site-specific management recommendations. We believe this distinction should be more precisely addressed, with tailored recommendations for key high-risk periorificial zones, particularly the periauricular region, midface (including the nose, upper lip, and labial rim), and periocular areas.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
FB: study design, article selection, review drafting and critical revision; AD: article selection, data extraction; GP, SS, AS, VM, DAM, VDS, MB, DC, CP, DR, EZ: article search and selection, critical revision; PN, AG, JG: critical revision of the article.
Ethical consideration
Not applicable.
History
Received: March 9, 2025
Accepted: March 18, 2025
Figures and tables
Figure 1. PRISMA 2020 flow diagram.
Figure 2. Typical early pattern of spread to the skin of nasal vestibule carcinomas between the alar and the lateral cartilages.
Figure 3. Primary squamous cell carcinoma of the ear with the characteristic infiltrative-erosive pattern of growth towards the external auditory canal.
| First author | Year | Country | Enrollment period | No. of patients | Age | Sex (M/F) | Tumour site | Nodal assessment | Histology | Surgery setting | Surgery type | Adjuvant treatment | Median FU (months) | Quality | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hamada 16 | 2005 | UK | 1987-2004 | 162 | 71.2* (33-97) | 77/85 | H zone | - | BCC | P | SR | - | 48.6 (2-120) | Fair | |
| Goh 17 | 2006 | Singapore | 1991-1995 | 166 | 70.9* (32-101) | 81/85 | H&N | - | BCC | P | SR | - | 74 | Fair | |
| Nemet 18 | 2006 | Australia | 1990-2004 | 469 | 68.3* (14-95) | 266/203 | H zone | 1+/468- | BCC + SCC | P+R | SR + PDEMA | 13/469 (2.8%) PORT | 33 (3-125) | Fair | |
| Maghami 19 | 2007 | USA | NA | 120 | 61§ (3-93) | 85/35 | H&N | 2+/118- | BCC + SCC | P+R | SR | 23/120 (19.1%) PORT; 1/120 (0.8%) | 27 (1-279) | Fair | |
| Auw-Haedrich 20 | 2009 | Germany | 1997-1999 | 101 | 65.5* (38-92) | 41/60 | H zone | - | BCC | P+R | SR | None | 87.6 (104-116.4) | Fair | |
| Kyrgidis 21 | 2009 | Greece | 1996-2006 | 1062 | 70.1* (15-97) | 620/442 | H&N | - | BCC | P | SR | - | 48 (12-144) | Fair | |
| Mourouzis 22 | 2009 | UK | 2000-2002 | 194 | 88* (72-104) | 143/51 | H&N | - | SCC | P | SR | 11/194 (5.7%) PORT | NA (30-60) | Fair | |
| Eskiizmir 23 | 2010 | Turkey | 2007-2008 | 21 | NA | NA | Nose | - | BCC + SCC | P | SR | 1/21 (4.8%) PORT | 28.5 (24-32) | Fair | |
| Bovill 14 | 2011 | UK | 2005-2006 | 79 | NA | NA | H&N | - | SCC | P+R | SR | - | 27.7 (24-64) | Fair | |
| Alaibac 25 | 2011 | Italy | 2003-2008 | 605 | 70* (28-95) | 356/249 | H&N | - | BCC | P+R | SR | - | NA (36-108) | Fair | |
| Goto 26 | 2012 | Japan | 1999-2008 | 218 | 73* | 104/114 | H&N | - | BCC | P | SR | - | 20.4 | Fair | |
| Najim 27 | 2012 | Australia | 1980-2010 | 102 | 63§ (17-97) | 75/27 | Lip | 3+/99- | SCC | P | SR | 25.5% PORT | - | Fair | |
| Essig 28 | 2013 | USA | 1995-2006 | 35 | 69§ (46-92) | 35/0 | Periauricular | 7+/22- | SCC | P+R | SR | 77% PORT | 36 (24-160) | Fair | |
| Bozan 29 | 2015 | Turkey | 2004-2014 | 154 | 74* (33-99) | 82/48 | H&N | - | BCC | P | SR | None | 69 (54-128) | Fair | |
| Armstrong 30 | 2016 | Australia | 2006-2007 | 331 | 66* (28-99) | 127/146 | H&N | - | BCC | P+R | SR | - | 70 | Fair | |
| Troeltzsch 31 | 2016 | Germany | 2008-2013 | 71 | 73.5* | 42/29 | H&N | - | BCC | P+R | SR | - | 43 | Fair | |
| Sullivan 32 | 2019 | USA | 2005-2016 | 43 | 74.7* | 34/9 | H&N | 8+/35- | SCC | P+R | SR | 28/43 (68.3%) PORT; 5/43 (12.2%) CT | 12 | Poor | |
| Galindo-Ferreiro 33 | 2020 | Spain | 2000-2016 | 337 | 69.4* | 175/162 | H zone | - | BCC + SCC | P | SR + PDEMA | - | - | Fair | |
| Fidelis 34 | 2021 | Brazil | 2008-2013 | 120 | 69.6* (31-100) | 60/60 | H&N | - | BCC | P+R | SR + PDEMA | - | 24 | Fair | |
| Ismail 35 | 2021 | Egypt | 2019-2021 | 40 | 56.4§ (38-79) | 27/13 | H&N | - | BCC | P+R | SR | - | 36 | Fair | |
| Komune 36 | 2021 | Japan | 1998-2019 | 71 | 66§ (37-88) | 28/43 | Periauricular | 13+/58- | SCC | P | SR | - | - | Fair | |
| Schachtel 37 | 2021 | Australia | 2000-2019 | 146 | 68.9§ (35-94) | 134/12 | Periauricular | 47+/98- | SCC | P+R | SR | 77.4% PORT | - | Good | |
| Altinel 38 | 2022 | Turkey | 2012-2021 | 649 | NA | NA | H&N | - | BCC | P | SR | None | > 12 | Fair | |
| Mendis 39 | 2022 | Australia | NA | 114 | 70* (34-91) | 92/22 | Scalp | - | SCC | P+R | SR | 28/114 (25%) PORT | 67.2 | Fair | |
| Ozbey 40 | 2022 | Turkey | 2016-2020 | 55 | 71.2* | 35/32 | H&N | - | BCC | P+R | SR | - | - | Fair | |
| Szewczyk 41 | 2022 | Polonia | 2008-2018 | 545 | 69* (26-100) | 304/241 | H&N | - | BCC | P+R | SR | - | - | Poor | |
| Tugrul 42 | 2022 | Turkey | 2000-2009 | 74 | 59.6* (31-91) | 59/15 | Lip | - | SCC | P+R | SR | 15/74 (20%) PORT | 42 (2-128) | Fair | |
| Goto 43 | 2023 | Japan | 2002-2021 | 46 | 67§ (34-84) | 24/22 | EAC | 8+/38- | SCC | P | SR | 13% PORT | 28 (2-224) | Good | |
| Komune 44 | 2023 | Japan | 2016-2022 | 40 | 67.5§ (33-83) | 15/25 | EAC | - | SCC | P+R | SR | 15% CRT | 25.3 (6.5-64.3) | Fair | |
| Dubray-Vautrin 45 | 2024 | France | 1999-2017 | 26 | 60.8* | 13/13 | EAC | 10+/16- | SCC | P | SR | 91.3% PORT; 7.7% CRT | 43 (8-108) | Good | |
| BCC: basal cell carcinoma; CT: chemotherapy; CRT: chemoradiotherapy; EAC: external auditory canal; FU: follow-up; H&N: head and neck; F: female; M: male; NA: not available;PORT: post-operative radiotherapy; P: primary; PDEMA: Peripheral Deep En Face Margin Assessment; R: recurrence; RT: radiotherapy; SCC: squamous cell carcinoma;SR: surgical resection. *mean; §median. | |||||||||||||||
| First author | SCC margin definition | BCC margin definition | Pathological margin | Positive margin (%) | Close margin (%) | LR | OS | DSS | RFS | LR time | Correlation margin/outcome | R1 predictors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1/LR | CM/LR | R1/prognosis | CM/prognosis | R1/RR | CM/RR | |||||||||||||
| Hamada 16 | NA | 4 mm | NA | 26/162 (16%) | NA | 4.35% (5y) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | NA | |
| Goh 17 | NA | 4 mm complete excision > 1mm | NA | 28/185 (15.1%) | NA | 1.6% (after re-excision) (74 m) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | Midface, female sex | |
| Nemet 18 | 5 mm | 3 mm | NA | 123/485 (25.4%) | NA | 27/485 (5.6%) | NA | NA | NA | 32 | Yes | NA | Yes | NA | NA | NA | High-risk histology (morpheaform) and location (medial canthus) | |
| Maghami 19 | NA | NA | NA | 24/120 (22.8%) | 3/120 (2.86%) | NA | 5y 64% | 5y 75% | NA | 18.5 | Yes | Yes | Yes | Yes | NA | NA | NA | |
| Auw-Haedrich 20 | NA | 2-3 solid BCC; 5 mm fibrous BCC | NA | 47/101 (46.5%) | 18/101 (17.8%) | 7/101 (6.9%) (83 m) | NA | NA | NA | NA | Yes | Yes | NA | NA | NA | NA | Fibrous BCC | |
| Kyrgidis 21 | NA | NA | < 2 mm close; > 2 mm negative | 72/1480 (5%) | 61/1480 T (4.1%) | 43/1062 (4%) | NA | NA | NA | NA | Yes | No | NA | NA | NA | NA | High-risk histology | |
| Mourouzis 22 | 5 mm | NA | Cells at the edge | 26/218 (11.9%) | NA | NA | NA | NA | NA | NA | NA | NA | Yes | NA | Yes | Yes | NA | |
| Eskiizmir 23 | 8-10 mm | 4-6 mm | NA | 3/21 (14.3%) | NA | 0/21 (after re-excision) 28.5 m | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | Perineural infiltration, infiltrative type BCC | |
| Bovill 24 | 4 mm | NA | NA | 21/79 (26.6%) | NA | 3/79 local (3.8%), 6/79 regional (7.6%) < 2y | NA | NA | NA | 12 | Yes | NA | No | NA | Yes | NA | NA | |
| Alaibac 25 | NA | NA | > 5 mm safe; < 5 mm unsafe | 122/719 T (16.9%) | NA | 105/719 (14.6%) | NA | NA | NA | NA | Yes | Yes | Yes | Yes | NA | NA | NA | |
| Goto 26 | NA | 3-5 mm | NA | 21/256 (8.2%) | NA | 0.7% (20.37 m) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | Nose, ears | |
| Najim 27 | NA | NA | NA | 28/102 (25.2%) | NA | 15/102 (14.8%) | NA | NA | NA | NA | NA | NA | Yes | Yes | NA | NA | NA | |
| Essig 28 | NA | NA | NA | 13/35 (37%) | NA | NA | 5y 49% | 5y 62% | NA | NA | Yes | NA | Yes | NA | NA | NA | NA | |
| Bozan 29 | NA | 4-7 mm | NA | 23/154 (14.9%) | NA | 10 (6.5%) | 5y 100% | NA | NA | NA | Yes | NA | No | NA | NA | NA | NA | |
| Armstrong 30 | NA | 3-5 mm | NA | 23/331 (6.9%) | NA | 10/331 (3%) (80 m) | NA | NA | NA | NA | Yes | Yes | NA | NA | NA | NA | Infiltrative and micronodular differentiation | |
| Troeltzsch 31 | NA | 3 mm | NA | 32/71 (45.1%) | NA | 11/71 (15.5%) | NA | NA | NA | NA | Yes | NA | Yes | NA | NA | NA | Tumour size > 2 cm, BBC in the nasal and perinasal areas. | |
| Sullivan 32 | NA | NA | < 1 mm close; > 1mm negative | 19/43 (44.2%) | 1/43 (2.3%) | NA | NA | NA | NA | NA | Yes | NA | Yes | NA | NA | NA | NA | |
| Galindo-Ferreiro 33 | NA | 2-3 mm well-defined borders; 5 mm ambiguous borders | NA | 69/337 (20.5%) | NA | 5.6% (19/337) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | Inner cantus | |
| Fidelis 34 | NA | 4 mm | NA | 34/120 (28.3%) | NA | 20% (29.5 m) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | Subsite (nasal and periocular) and sex (female) | |
| Ismail 35 | NA | 3 mm | NA | 9/40 (22.5%) | NA | 9/40 (22.5%) | NA | NA | 3y 68% | 6 | Yes | NA | Yes | NA | Yes | No | NA | |
| Komune 36 | NA | NA | NA | 11/51 (21.6%) | NA | NA | NA | NA | NA | NA | NA | NA | Yes | NA | NA | NA | NA | |
| Schachtel 37 | > 10 mm for the main tumour > 5 mm in nerves with PNS | NA | NA | 46/146 (31.5%) | 52/146 (35.6%) | NA | 5y 44.7% | 5y 67.9% | NA | 14.3 (1-60) | Yes | Yes | Yes | Yes | NA | NA | NA | |
| Altinel 38 | NA | 1-2 mm H area; 3-5 mm other sites | NA | 78/649 (12%) | NA | 0/78 (0%) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | NA | |
| Mendis 39 | NA | NA | < 2 mm incomplete excision | 62/114 (56%) | NA | 23/114 (20%) | NA | NA | NA | NA | Yes | Yes | Yes | Yes | NA | NA | NA | |
| Ozbey 40 | NA | NA | < 1 mm close; > 1 mm negative | 6/67 T (9%) | 11/67 (16.4%) | 1/67 (1.5%) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | NA | |
| Szewczyk 41 | NA | NA | NA | 107/545 (19.6%) | NA | 52/545 (9.5%) | NA | NA | NA | NA | Yes | NA | NA | NA | NA | NA | NA | |
| Tugrul 42 | NA | NA | < 0.5 mm close; > 0.5 mm negative | 5/74 (7%) | 26/74 (36%) | 5/74 (6.7%) | 3y 90%; 5y 80% | NA | NA | NA | No | NA | NA | NA | NA | NA | NA | |
| Goto 43 | NA | NA | NA | 4/30 (13%) | NA | NA | 5y 69.5% | 5y 77.6% | NA | NA | NA | NA | No | NA | NA | NA | NA | |
| Komune 44 | NA | NA | NA | 9/40 (22.5%) | NA | NA | 2y 85.2% | 2y 88.8% | NA | NA | Yes | NA | Yes | NA | NA | NA | NA | |
| Dubray-Vautrin 45 | NA | NA | NA | 8/23 (34.8%) | NA | NA | 5y 64.5% | NA | NA | NA | NA | NA | Yes | NA | NA | NA | NA | |
| BCC: basal cell carcinoma; CM: close margins; DSS: disease-specific survival; LR: local recurrence; m: months; NA: not available; OS: overall survival; R0: negative margin; R1: positive margin; RFS: recurrence-free survival; RR: regional recurrence; SCC: squamous cell carcinoma; y: years. | ||||||||||||||||||
References
- Rogers H, Weinstock M, Feldman S. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086. doi:https://doi.org/10.1001/jamadermatol.2015.1187
- Aggarwal P, Knabel P, Fleischer A. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:https://doi.org/10.1016/j.jaad.2021.03.109
- Global Burden of Disease Cancer Collaboration et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2019;5:1749-1768. doi:https://doi.org/10.1001/jamaoncol.2019.2996
- Quazi S, Aslam N, Saleem H. Surgical margin of excision in basal cell carcinoma: a systematic review of literature. Cureus. 2020;12. doi:https://doi.org/10.7759/cureus.9211
- Wolf D, Zitelli J. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Bussu F, Tagliaferri L, Crescio C. New standards for the management of nose vestibule malignancies. Acta Otolaryngol. 2023;143:215-222. doi:https://doi.org/10.1080/00016489.2023.2179662
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Squamous Cell Skin Cancer Version 2.2025.
- Tagliaferri L, Giarrizzo I, Fionda B. ORIFICE (Interventional Radiotherapy for Face Aesthetic Preservation) study: results of interdisciplinary assessment of interstitial interventional radiotherapy (brachytherapy) for periorificial face cancer. J Pers Med. 2022;12. doi:https://doi.org/10.3390/jpm12071038
- Shuber E, Abdulhussein D, Sinclair P. Who should carry out skin cancer excisions? A systematic review. J Cutan Aesthet Surg. 2019;12:153-157. doi:https://doi.org/10.4103/JCAS.JCAS_174_18
- Bittner G, Cerci F, Kubo E. Mohs micrographic surgery: a review of indications, technique, outcomes, and considerations. An Bras Dermatol. 2021;96:263-277. doi:https://doi.org/10.1016/j.abd.2020.10.004
- Golda N, Hruza G. Mohs micrographic surgery. Dermatol Clin. 2023;41:39-47. doi:https://doi.org/10.1016/j.det.2022.07.006
- Zürcher S, Martignoni Z, Hunger R. Mohs micrographic surgery for cutaneous squamous cell carcinoma. Cancers (Basel). 2024;16. doi:https://doi.org/10.3390/cancers16132394
- Booth A, Clarke M, Ghersi D. An international registry of systematic-review protocols. Lancet. 2011;377:108-109. doi:https://doi.org/10.1016/S0140-6736(10)60903-8
- Page M, McKenzie J, Bossuyt P. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. doi:https://doi.org/10.1136/bmj.n71
- Study Quality Assessment Tools|NHLBI, NIH.
- Hamada S. Eyelid basal cell carcinoma: non-Mohs excision, repair, and outcome. Brit J Ophthalmol. 2005;89:992-994. doi:https://doi.org/10.1136/bjo.2004.058834
- Goh B, Ang P, Wu Y. Characteristics of basal cell carcinoma amongst Asians in Singapore and a comparison between completely and incompletely excised tumors. Int J Dermatology. 2006;45:561-564. doi:https://doi.org/10.1111/j.1365-4632.2004.02515.x
- Nemet A, Deckel Y, Martin P. Management of periocular basal and squamous cell carcinoma: a series of 485 cases. Am J Ophthalmol. 2006;142:293-297. doi:https://doi.org/10.1016/j.ajo.2006.03.055
- Maghami E, Talbot S, Patel S. Craniofacial surgery for nonmelanoma skin malignancy: Report of an international collaborative study. Head & Neck. 2007;29:1136-1143. doi:https://doi.org/10.1002/hed.20656
- Auw-Haedrich C, Frick S, Boehringer D. Histologic safety margin in basal cell carcinoma of the eyelid. Ophthalmology. 2009;116:802-806. doi:https://doi.org/10.1016/j.ophtha.2008.11.012
- Kyrgidis A, Vahtsevanos K, Tzellos T. Clinical, histological and demographic predictors for recurrence and second primary tumours of head and neck basal cell carcinoma. A 1062 patient-cohort study from a tertiary cancer referral hospital. Eur J Dermatol. 2010;20:276-282. doi:https://doi.org/10.1684/ejd.2010.0903
- Mourouzis C, Boynton A, Grant J. Cutaneous head and neck SCCs and risk of nodal metastasis – UK experience. J Craniomaxillofac Surg. 2009;37:443-447. doi:https://doi.org/10.1016/j.jcms.2009.07.007
- Eskiizmir G, Gençoğlan G, Temiz P. Staged-surgery with permanent pathology for the management of high-risk nonmelanoma skin cancer of the nose. Eur Arch Otorhinolaryngol. 2011;268:117-121. doi:https://doi.org/10.1007/s00405-010-1324-x
- Bovill E, Banwell P. Re-excision of incompletely excised cutaneous squamous cell carcinoma: histological findings influence prognosis. J Plast Reconstr Aesthet Surg. 2012;65:1390-1395. doi:https://doi.org/10.1016/j.bjps.2012.04.031
- Alaibac M. Facial basal cell carcinoma: analysis of recurrence and follow-up strategies. Oncol Rep. 2011;26:1423-1429. doi:https://doi.org/10.3892/or.2011.1453
- Goto M, Kai Y, Arakawa S. Analysis of 256 cases of basal cell carcinoma after either one-step or two-step surgery in a Japanese institution. J Dermatol. 2012;39:68-71. doi:https://doi.org/10.1111/j.1346-8138.2011.01306.x
- Najim M, Cross S, Gebski V. Early-stage squamous cell carcinoma of the lip: the Australian experience and the benefits of radiotherapy in improving outcome in high-risk patients after resection. Head Neck. 2013;35:1426-1430. doi:https://doi.org/10.1002/hed.23148
- Essig G, Kithipornchai L, Adams F. Lateral temporal bone resection in advanced cutaneous squamous cell carcinoma: report of 35 patients. J Neurol Surg B Skull Base. 2013;74:54-59. doi:https://doi.org/10.1055/s-0032-1331021
- Bozan A, Gode S, Kaya I. Longterm follow-up of positive surgical margins in basal cell carcinoma of the face. Dermatologic Surg. 2015;41:761-767. doi:https://doi.org/10.1097/DSS.0000000000000394
- Armstrong L, Magnusson M, Guppy M. The role of embryologic fusion planes in the invasiveness and recurrence of basal cell carcinoma: a classic mix-up of causation and correlation. Plast Reconstr Surg Glob Open. 2015;3. doi:https://doi.org/10.1097/GOX.0000000000000571
- Troeltzsch M, Probst F, Knosel T. Clinical and pathologic parameters predicting recurrence of facial basal cell carcinoma: a retrospective audit in an advanced care center. Int J Dermatol. 2016;55:1281-1288. doi:https://doi.org/10.1111/ijd.13341
- Sullivan C, Andresen N, Kendell N. Survival outcomes for advanced cutaneous squamous cell carcinoma of the head and neck. Ann Otol Rhinol Laryngol. 2019;128:949-955. doi:https://doi.org/10.1177/0003489419848786
- Galindo-Ferreiro A, Sanchez-Tocino H, Diez-Montero C. Characteristics and recurrence of primary eyelid basal cell carcinoma in Central Spain. J Curr Ophthalmol. 2020;32:183-188. doi:https://doi.org/10.4103/JOCO.JOCO_28_20
- Fidelis M, Stelini R, Staffa L. Basal cell carcinoma with compromised margins: retrospective study of management, evolution, and prognosis. An Bras Dermatol. 2021;96:17-26. doi:https://doi.org/10.1016/j.abd.2020.11.001
- Ismail A, Abdelwabab M, Yassin M. Evaluation of the best surgical margin for basal cell carcinoma. Egypt J Hosp Med. 2021;83:1618-1628.
- Komune N, Noda T, Kogo R. Primary advanced squamous cell carcinoma of the temporal bone: a single-center clinical study. The Laryngoscope. 2021;131. doi:https://doi.org/10.1002/lary.28653
- Schachtel M, Gandhi M, Bowman J. Epidemiology and treatment outcomes of cutaneous squamous cell carcinoma extending to the temporal bone. Head Neck. 2022;44:2727-2743. doi:https://doi.org/10.1002/hed.27185
- Serin M, Altinel D, Toplu G. Requirement of re-excision in surgical margin positive basal cell carcinoma cases without macroscopic residual lesions (our experience of 714 cases and a review of the literature). Turk J Anesth Reanim. 2022;16. doi:https://doi.org/10.4103/tjd.tjd_45_22
- Mendis R, Morgan G, Abdul-Razak M. Margin status predicts outcome in patients with cutaneous squamous cell carcinoma of the scalp: the Westmead hospital experience. Skin J Cutan Med. 2022;6:295-303. doi:https://doi.org/10.25251/skin.6.4.3
- Ozbey R. Basal cell skin cancers: retrospective analysis of 67 cases. J Cosmetic Dermatol. 2022;21:7007-7012. doi:https://doi.org/10.1111/jocd.15441
- Szewczyk M, Pazdrowski J, Pabiszczak M. Local recurrence risk in head and neck basal cell carcinoma. Otolaryngol Pol. 2022;76:1-5. doi:https://doi.org/10.5604/01.3001.0015.8568
- Tugrul F, Isık N, Yaprak G. Factors affecting the recurrence of early-stage lip cancer. Int J Radiat Res. 2022;20:323-327. doi:https://doi.org/10.52547/ijrr.20.2.10
- Goto S, Nishio N, Iwami K. Surgical strategy for squamous cell carcinoma of the external auditory canal: management of locally advanced cases with skull base involvement. J Neurol Surg B Skull Base. 2023;84:69-78. doi:https://doi.org/10.1055/a-1733-2585
- Komune N, Kuga R, Hongo T. Impact of positive-margin resection of external auditory canal squamous cell carcinoma. Cancers (Basel). 2023;15. doi:https://doi.org/10.3390/cancers15174289
- Dubray-Vautrin A, Vérillaud B, Herman P. Squamous cell carcinoma of the temporal bone: the impact of local control on survival. Acta Otolaryngol. 2025;145:23-29. doi:https://doi.org/10.1080/00016489.2024.2311788
- Diepgen T, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146:1-6. doi:https://doi.org/10.1046/j.1365-2133.146
- Niculet E, Craescu M, Rebegea L. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches. Exp Ther Med. 2022;23. doi:https://doi.org/10.3892/etm.2021.10982
- Khalil A, Enezei H, Aldelaimi T. Advances in diagnosis and treatment of basal cell carcinoma. J Craniofac Surg. 2024;35:E204-E208. doi:https://doi.org/10.1097/SCS.0000000000009959
- Goh K-I, Ghim C, Kahng B. Gohet al. reply. Phys Rev Lett. 2003;91.
- Català A, Garces J, Alegre M. Mohs micrographic surgery for basal cell carcinomas: results of a Spanish retrospective study and Kaplan-Meier survival analysis of tumor recurrence. J Eur Acad Dermatol Venereol. 2014;28:1363-1369. doi:https://doi.org/10.1111/jdv.12293
- Morgan F, Ruiz S, Karia P. Factors predictive of recurrence, metastasis, and death from primary basal cell carcinoma 2 cm or larger in diameter. J Am Acad Dermatol. 2020;83:832-838. doi:https://doi.org/10.1016/j.jaad.2019.09.075
- Kim J, Kozlow J, Mittal B. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540-559. doi:https://doi.org/10.1016/j.jaad.2017.10.006
- Peris K, Fargnoli M, Garbe C. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:https://doi.org/10.1016/j.ejca.2019.06.003
- Mutimer C, Dicker A. Marking a surgical margin for excision of a keratinocyte cancer. Aust J Gen Pract. 2021;50:385-387. doi:https://doi.org/10.31128/AJGP-12-19-5171
- Kimyai-Asadi A, Alam M, Goldberg L. Efficacy of narrow-margin excision of well-demarcated primary facial basal cell carcinomas. J Am Acad Dermatol. 2005;53:464-468. doi:https://doi.org/10.1016/j.jaad.2005.03.038
- Uhlman K, Bonert M, Yuen K. Margin status of basal cell carcinoma: what can be done better?. J Plast Reconstr Aesthet Surg. 2024;97:156-162. doi:https://doi.org/10.1016/j.bjps.2024.07.063
- Laga A, Schaefer I, Sholl L. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152:706-717. doi:https://doi.org/10.1093/ajcp/aqz089
- Gellatly M, Cruzval-O’Reilly E, Mervak J. Metastatic basal cell carcinoma with atypical pattern of spread. Radiol Case Rep. 2020;15:2641-2644. doi:https://doi.org/10.1016/j.radcr.2020.09.054
- Lo J, Snow S, Reizner G. Metastatic basal cell carcinoma: report of twelve cases with a review of the literature. J Am Acad Dermatol. 1991;24:715-719. doi:https://doi.org/10.1016/0190-9622(91)70108-e
- Yildiz P, Aung P, Milton D. Measurement of tumor thickness in cutaneous squamous cell carcinomas: do the different methods provide better prognostic data?. Am J Dermatopathol. 2020;42:337-342. doi:https://doi.org/10.1097/DAD.0000000000001511
- Veness M, Porceddu S, Palme C. Cutaneous head and neck squamous cell carcinoma metastatic to parotid and cervical lymph nodes. Head Neck. 2007;29:621-631. doi:https://doi.org/10.1002/hed.20576
- Vauterin T, Veness M, Morgan G. Patterns of lymph node spread of cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2006;28:785-791. doi:https://doi.org/10.1002/hed.20417
- Gurudutt V, Genden E. Cutaneous squamous cell carcinoma of the head and neck. J Skin Cancer. 2011;2011. doi:https://doi.org/10.1155/2011/502723
- Bussu F, Tagliaferri L, Piras A. Multidisciplinary approach to nose vestibule malignancies: setting new standards. Acta Otorhinolaryngol Ital. 2021;41:S158-S165. doi:https://doi.org/10.14639/0392-100X-suppl.1-41-2021-16
- Bussu F, Rizzo D, Tramaloni P. Malignancies of the Nasal Vestibule. Springer International Publishing; 2023.
- Scaglione G. Malignancies of the Nasal Vestibule. Springer International Publishing; 2023.
- Testa G, Mattavelli D, Rampinelli V. Squamous cell carcinoma of the nasal vestibule: a diagnostic and therapeutic challenge. Eur Arch Otorhinolaryngol. 2024;281:5627-5640. doi:https://doi.org/10.1007/s00405-024-08813-8
- Bussu F, Tagliaferri L, Mattiucci G. Comparison of interstitial brachytherapy and surgery as primary treatments for nasal vestibule carcinomas. Laryngoscope. 2016;126:367-371. doi:https://doi.org/10.1002/lary.25498
- Bussu F, Tagliaferro L, Corbisiero M. Management of nasal vestibule carcinomas: recommendations by the Oncological Committee of the Italian Society of Otorhinolaryngology – Head and Neck Surgery. Acta Otorhinolaryngol Ital. 2024;44:13-20. doi:https://doi.org/10.14639/0392-100X-N2786
- Bussu F. Malignancies of the nasal vestibule: a new perspective on classification, staging, and treatment. Springer Nature. Published online 2023.
- Tropiano P. From applicator-based (Paris system) implantations to rhinoseptoplasty: the concept of anatomic implantation for interventional radiotherapy in squamous cell carcinoma of the nasal vestibule. Short term results in a monoinstitutional series. Mini-invasive Surg. Published online 2024. doi:https://doi.org/10.20517/2574-1225.2024.41
- Guinot J, Bacorro W, Budrukkar A. GEC-ESTRO recommendations for head & neck cancer brachytherapy (interventional radiotherapy): 2nd update with focus on HDR and PDR. Radiother Oncol. 2024;201. doi:https://doi.org/10.1016/j.radonc.2024.110533
- Tagliaferri L, Ciardo F, Fionda B. Non-melanoma skin cancer treated by contact high-dose-rate radiotherapy (brachytherapy): a mono-institutional series and literature review. In Vivo. 2021;35:2313-2319. doi:https://doi.org/10.21873/invivo.12505
- Gonzalez-Perez V, Rambielak A, Guinot J. H&N and Skin (HNS) GEC-ESTRO Working Group critical review of recommendations regarding prescription depth, bolus thickness and maximum dose in skin superficial brachytherapy with flaps and customized moulds. Radiother Oncol. 2022;175:122-132. doi:https://doi.org/10.1016/j.radonc.2022.08.022
- Zanoletti E, Marioni G, Stritoni P. Temporal bone squamous cell carcinoma: analyzing prognosis with univariate and multivariate models. Laryngoscope. 2014;124:1192-1198. doi:https://doi.org/10.1002/lary.24400
- Komune N, Kuga D, Matsuo S. Clinical analysis of en bloc resection for advanced temporal bone squamous cell carcinoma. J Neurol Surg B Skull Base. 2022;83:E40-E48. doi:https://doi.org/10.1055/s-0041-1722930
- Zanoletti E, Tealdo G, Franz L. From ‘Extended total parotidectomy’ to ‘Temporo-parotid resection’ for locally advanced parotid tumors: outlining a shift in surgical perspective. Oral Oncol. 2022;131. doi:https://doi.org/10.1016/j.oraloncology.2022.105975
- Shao A, Wong D, Mclvor N. Parotid metastatic disease from cutaneous squamous cell carcinoma: prognostic role of facial nerve sacrifice, lateral temporal bone resection, immune status and P-stage. Head Neck. 2014;36:545-550. doi:https://doi.org/10.1002/hed.23323
- Coombs A, Butler A, Allison R. Metastatic cutaneous squamous cell carcinoma of the parotid gland: prognostic factors. J Laryngol Otol. 2018;132:264-269. doi:https://doi.org/10.1017/S0022215117001323
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1354 times
- PDF downloaded - 429 times



