Head and neck
Vol. 45: 111TH CONGRESS SIOECHCF - OFFICIAL REPORT 2025
Surgical margin assessment and prognostic impact in sinonasal cancers: a systematic review and meta-analysis
Abstract
Objective. Surgery remains a cornerstone in treatment of sinonasal malignancies, but the prognostic role of margin status is controversial. This systematic review and meta-analysis evaluated the prognostic significance of surgical margins in sinonasal cancer and their impact on survival, alongside key challenges in its evaluation.
Methods. A systematic search in PubMed, Scopus, and Web of Science identified 64 studies (34,120 patients).
Results. The overall margin infiltration rate was 33.2%, varying widely across studies (4.5-88.2%) and histotypes, and was the highest in adenoid cystic carcinoma (ACC, 61.5%). Meta-analysis of 31 studies showed positive margins were associated with worse survival (overall survival, odds ratio [OR] 2.61; disease-specific survival, OR 5.89; disease-free survival, OR 4.40). Squamous cell carcinoma, olfactory neuroblastoma, and mucosal melanoma had the strongest correlation with margin status, while for ACC and adenocarcinomas statistical significance was not reached. High heterogeneity was noted across studies, alongside inconsistent margin classification, distance thresholds, and use of frozen sections, limiting cross-study comparability.
Conclusions. This study confirms the prognostic value of surgical margins, but underscores the urgent need for standardised definitions to improve prediction of oncologic outcomes and clinical decision-making.
Introduction
Sinonasal tumours are a rare and heterogeneous group of malignancies with multiple histopathological subtypes. Over the past decades, treatment strategies have significantly evolved, driven by advances in multimodal protocols tailored to tumour histology 1. However, no universally accepted treatment guidelines exist, as therapeutic approaches continue to adapt alongside an evolving understanding of tumour biology 2. While non-surgical strategies, including neoadjuvant chemotherapy and immunotherapy, hold promise for expanding future treatment options, surgery remains the cornerstone for most resectable tumours and has been the primary therapeutic approach in recent decades 3.
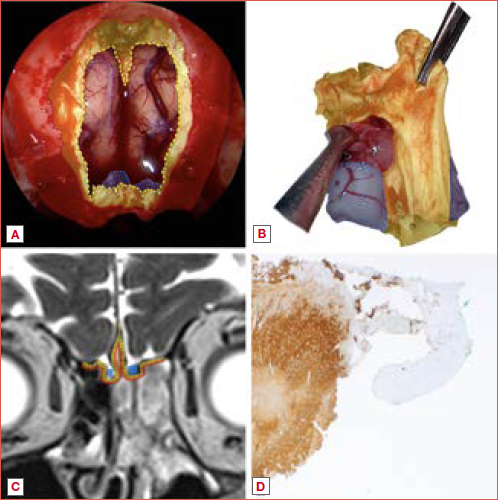
Surgical margin status is a key determinant of oncologic outcomes, influencing local control, recurrence rates, overall survival, and the need for adjuvant treatment. Achieving clear margins in sinonasal tumours is particularly challenging due to the complex anatomy and proximity to critical structures, such as the skull base, orbit, major vessels, and cranial nerves. Over the past two decades, endoscopic techniques have transformed the surgical landscape, providing superior visualisation, reduced morbidity, and improved functional outcomes compared to open approaches 3-6. However, these techniques have also complicated the definition of complete resection. Since en-bloc tumour removal is often unfeasible, oriented multi-bloc resection has emerged as an oncologically-safe alternative, requiring meticulous technique to preserve tumour orientation and spatial relationships and ensure accurate margin evaluation (Cover figure) 2,7.
Despite the recognised importance of surgical margins, no universally accepted definition of a clear margin exists for sinonasal tumours 4. This lack of consensus complicates the interpretation of surgical outcomes and hinders cross-study comparisons. Additionally, heterogeneity in the literature – with variations in sample size, histological subtypes, and study design – further limits the ability to establish a solid prognostic role for margin involvement. Given these challenges, a comprehensive evaluation of existing evidence is warranted to clarify the impact of surgical margins on survival outcomes and to assess the methodologies used for margin evaluation.
This systematic review and meta-analysis aims to summarise the available evidence on the definition and prognostic significance of surgical margins in sinonasal cancer, evaluating their impact on survival outcomes and the methodologies used for margin assessment.
Materials and methods
Protocol registration
The protocol of this systematic review and meta-analysis was registered on PROSPERO, an international database of prospectively registered systematic reviews in health and social care (Center for Reviews and Dissemination, University of York, York, UK), on November 24, 2024, under the registry number CRD42024618259.
Search strategy
A systematic literature review was performed in alignment with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 8. The inclusion criteria for study selection were defined according to the PICOS framework: patients (P), adults diagnosed with sinonasal malignancies; intervention (I), surgical treatment performed via open or endoscopic approaches; comparator (C), none; outcomes (O), overall survival (OS), disease-specific survival (DSS), disease-free survival (DFS), local recurrence-free survival (LRFS); study design (S), retrospective and prospective cohort studies.
A comprehensive literature search was conducted in PubMed, Scopus, and Web of Science, using predefined search strings tailored for each database, from database inception to 21 November 2024. The search strategy included terms related to sinonasal and paranasal anatomical region, neoplasms, surgical margins, and prognostic outcomes. The full search queries are available in Supplementary Table SI. No filters or language restrictions were applied. The reference lists of all included articles were thoroughly screened to identify additional relevant studies.
References were exported to Zotero (v6.0.10, Center for History and New Media, George Mason University, Fairfax, VA, USA) for bibliography management. After duplicate removal, a team of 3 reviewers (ADA, EC, GM) independently screened all titles and abstracts, followed by a full-text evaluation of eligible articles based on the inclusion criteria. Any disagreements were resolved through discussion among all authors to reach a consensus.
No ethical approval or informed consent were required for this study, as all reported data were obtained from publicly available published literature.
Eligibility criteria
The inclusion criteria for the studies were: (a) histopathological diagnosis of sinonasal malignancy; (b) treated with an open or endoscopic surgical approach; (c) surgical margin status clearly reported, using any defined classification, including clinical or pathological assessment, microscopic or macroscopic infiltration, or close margins; (d) availability of at least one survival outcome, including OS, DSS, DFS, LRFS, regional recurrence-free survival (RRFS), or distant recurrence-free survival (DRFS); (e) correlation between survival outcome and margin status, requiring studies to either provide survival data stratified by margin status or analyse the impact of margin status on survival using univariate or multivariate analysis.
The exclusion criteria were: (a) unavailability of the full text; (b) overlapping cohorts or data redundancy, in which case the most recent and comprehensive eligible publication was selected; (c) absence of relevant clinicopathological data regarding margin status and survival outcomes; (d) studies reporting fewer than 10 cases of interest; (e) inability to segregate data between surgical and non-surgical cohorts; (f) articles including survival data on sarcomas, tumours of uncertain malignant potential, haematolymphoid malignancies, or tumours originating from extra-nasal sites, unless survival data for sinonasal malignancies of interest were separately analysable; (g) non-original studies, such as letters, editorials, and book chapters; (h) animal studies; (i) non-English publications.
In cases where eligible articles did not provide the necessary data within the text, the corresponding authors were contacted to request raw data. Articles were included if the requested data were provided; otherwise, they were excluded from the analysis. All papers were thoroughly screened for duplicates.
Data extraction
Two independent reviewers (EC and GS) extracted data from the included studies using a standardised data collection form. Discrepancies were resolved through discussion or, if necessary, by consulting a third reviewer (ADA). The data extracted included study characteristics (author, year of publication, country, enrollment period), patient demographics (total number of patients, age, gender, histopathological diagnosis, primary tumour site, tumour staging), treatment strategies (patients treated surgically, type of surgery performed, prior treatments and use of adjuvant therapy in the surgical cohort), surgical margin status, survival outcomes, and follow-up duration.
Surgical margin status was extracted as reported by the authors. Additionally, an analysis of the methods used to define margins was conducted, including any threshold distance in millimetres, the use of frozen sections, and the distinction between close margins, microscopic infiltration, or macroscopic involvement. Furthermore, any other qualitative descriptions of how margins were assessed and managed by the authors were also examined.
Quality assessment
The quality of the studies eligible for inclusion was categorised as poor, fair or good, in agreement with the National Institutes of Health quality assessment tool for Observational Cohorts and Cross-Sectional Studies (NHLBI NIH). Study quality assessment tools. July 2021. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed December 10, 2024). Three reviewers (ADA, AL and GD) independently evaluated the papers, and any disagreements were resolved by discussion.
Data synthesis
Data analysis was conducted using both quantitative and qualitative synthesis, depending on the availability and structure of the reported outcomes. Studies were eligible for quantitative analysis (meta-analysis) if they provided 5-year survival data stratified by positive and negative margins, explicitly reporting the number of patients alive at 5 years in relation to OS, DSS, and DFS. These studies were included in the pooled statistical analysis to estimate the impact of margin status on survival. Where feasible, subgroup analyses were performed based on histology.
As a secondary outcome, studies were included in the qualitative synthesis if they assessed the impact of margin status on any survival endpoint (OS, DSS, DFS, LRFS, RRFS, and DRFS) through uni- or multivariate analysis but did not provide exact survival percentages for each group. While these studies were not eligible for meta-analysis, they were considered in the overall evaluation of the prognostic role of surgical margins and analysed descriptively.
Statistical analysis
The meta-analysis was conducted using RStudio (version 2024.12.0+467, Posit Software, PBC). Event rates were calculated for the following 5-year outcomes: OS, considering death from any cause as an event; DSS, considering disease-specific death as an event; and DFS, considering disease recurrence or death from any cause as an event.
To assess the association between margin status and survival outcomes, the odds ratio (OR) of event rates was calculated, comparing positive and negative margin groups. A pooled OR with a 95% confidence interval (CI) was estimated using the DerSimonian and Laird random-effects model. The p value was derived using a Z-test based on the pooled OR and its standard error. Heterogeneity among studies was evaluated using Cochran’s Q test and the I2 statistic. A p value < 0.05 in the Q test or an I2 > 50% was considered indicative of substantial heterogeneity. Potential publication bias was assessed using funnel plots and Egger’s linear regression test, with a p value < 0.05 indicating statistically significant asymmetry.
Results
Literature search and study identification
A total of 3,638 records were initially identified through the combined database search. After duplicate removal and initial screening, 2,459 titles were assessed for eligibility. Following the application of inclusion and exclusion criteria, 64 studies 9-72 were included in the qualitative synthesis, of which 31 were deemed eligible for the quantitative analysis 11,12,14,19,21,22,24,27,30,31,33-39,41,44,46-48,50,51,55-57,64,68,71,72. A detailed PRISMA flowchart illustrating the search process is presented in Figure 1.
Quality assessment
According to the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, 38 (59.4%) studies 10,12,13,15,16,20,22-24,27-40,42,44-47,53,54,57,59,60,62,67,69-71 were classified as good quality, while 26 (40.6%) 9,11,14,17-19,21,25,26,41,43,48-52,55,56,58,61,63-66,68,72 were rated as fair. No studies were deemed poor, as none exhibited a significant lack of clinical details (Tab. I).
Characteristics of the studies included
Among the 64 studies included, 46 were retrospective case series (RCS) and 18 were National Cancer Database (NCDB) analyses. These studies were published over a 25-year period (1999-2024), with patient recruitment spanning from 1956 to 2022. The majority of the studies were from Northern America, Europe, and Asia, with a few contributions from Australia.
The total number of patients reported across all studies was 34,120, with individual sample sizes ranging from 12 to 7,808 patients. The mean patient age varied between 44 and 71 years, with a male predominance reported in most studies.
Regarding histological distribution, squamous cell carcinoma (SCC) was the most frequent histotype, accounting for 18,859 cases (64.6%). Olfactory neuroblastoma (ONB) followed with 3,437 cases (11.8%), while sinonasal mucosal melanoma (SNMM) was reported in 3,527 cases (12.1%). Sinonasal adenocarcinoma (SNAC) accounted for 1,137 cases (3.9%), and sinonasal undifferentiated carcinoma (SNUC) for 740 cases (2.5%). Adenoid cystic carcinoma (ACC) was identified in 817 cases (2.8%), whereas minor salivary glands tumours (MSGT) were observed in 458 cases (1.6%). Less frequent histotypes included sinonasal neuroendocrine carcinoma (SNEC) with 95 cases (0.3%). Other rare histotypes were reported in 118 cases (0.4%).
The surgical approach was detailed in 43 of 68 studies, while in 21 the type of surgery was not specified. Among the reported cases, 2,662 patients underwent endoscopic surgery, whereas 3,483 patients were treated with an open or combined approach. The follow-up period varied significantly among studies, ranging from 0 to 288 months. Patient and tumour characteristics of the studies included are summarised in Table II.
Regarding margin definition, most studies lacked a specific description of their assessment (Tab. III). Margin status was reported in all studies but not for all patients, covering a total of 17,758 patients. Among them, 11,806 patients had negative margins, 5,899 patients had positive margins, and 53 patients were classified as having close margins. The pooled global rate of margin infiltration was 33.2% with a wide range, spanning from 4.5% to 88.2%. Notably, median rates of margin infiltration varied considerably across histotypes. Focusing on 54 articles analysing single histological groups, SCC showed a median infiltration rate of 31.4% (17.2-65.9%), while ONB had a median rate of 29% (4.5-56.8%). SNAC presented a higher median infiltration rate of 42.9% (21.1-45.5%), whereas SNMM exhibited a median rate of 31.3% (13.9-50%). Among salivary-type malignancies, ACC displayed a notably high infiltration rate, with a median of 61.5% (61.1-88.2%), while other salivary tumour had a median of 27.4% (22.2-32.5%). Lastly, SNUC demonstrated a median infiltration rate of 43.4% (37.3-49.5%).
Qualitative analysis
The studies were evaluated for any reported associations between margin status and survival, based on uni- and multivariate analyses of the previously mentioned survival outcomes in each study. Among the 64 included papers, 10 (15.6%) evaluated multiple histotypes, of which 8 (80%) identified at least one significant association between margin status and survival. The remaining 54 studies (84.4%) focused on a single histology, of which 36 (66.7%) reported a significant association, while 18 (33.3%) did not find a significant correlation. When considering SNMM, a total of 3,145 patients were analysed across 6 studies identifying at least one significant association. For ONB, 2,906 patients were included across 13 studies with significant findings. Regarding SCC, 17,921 patients were analysed in 12 studies showing a significant association. In the case of SNAC, 123 patients were evaluated across 3 studies, while for SNUC, 173 patients were analysed in one study with significant results. Lastly, one study on MSGT, including 239 patients, identified a significant association. Details regarding specific survival outcomes and statistical models used are shown in Table II.
Meta-analysis
Clinical and methodological heterogeneity precluded further statistical analysis and meta-analysis of LRFS. A total of 35 studies reported outcomes stratified by margin status for OS, DSS, or DFS (Fig. 2). After excluding 5 studies 25,40,42,52,61 due to the absence of data on 5-year outcomes, 28 were eligible for quantitative analysis of the association between margin status and OS 11,12,14,19,21,22,24,27,30,31,33,34,37-39,41,44,46-48,50,55-57,64,68,71,72, 5 for DSS 22,35,36,47,48, and 6 for DFS 14,22,24,27,44,48.
The meta-analysis of 28 studies assessing the impact of surgical margin status on OS irrespective of histotype revealed a significant association between positive margins (R1) and poorer survival outcomes. The pooled OR for 5-year OS was 2.61 (95% CI: 1.98-3.44, p < 0.001), indicating that patients with R1 resections had a significantly higher risk of mortality compared to those with negative margins. A uniform direction of effect was found across all studies, although only 10 studies individually reached statistical significance. Heterogeneity was substantial (I2 = 69%, τ2=0.2263, p < 0.01), suggesting variability across studies.
The meta-analysis of studies assessing the impact of surgical margin status on DSS, irrespective of histotype, demonstrated a significant association between positive margins (R1) and worse survival outcomes. The pooled OR for 5-year DSS was 5.89 (95% CI: 4.22-8.22, p < 0.001), indicating that patients with R1 resections had a significantly higher risk of disease-related mortality compared to those with negative margins. Unlike OS, heterogeneity was low (I2 = 0%, p = 0.64), suggesting a high degree of consistency across studies. A uniform direction of effect was observed, although only 2 studies individually reached statistical significance.
A similar result was found in the meta-analysis assessing DFS, with a pooled OR for 5-year DFS of 4.4 (95% CI: 2.12-9.11, p < 0.001) and moderate heterogeneity across studies (I2 = 47%, p = 0.09). Only 2 studies individually reached statistical significance.
When stratified by histology, a significant association between negative margins and 5-year OS was found in SNMM (OR 4.89, 95% CI: 1.41-17, p = 0.013), SCC (OR 3.77, 95% CI: 1.52-9.4, p = 0.004), and ONB (OR 6.85, 95% CI: 2.6-18.09, p < 0.001). Conversely, statistical significance was not reached for ACC (OR 2.43, 95% CI: 0.53-11.19, p = 0.253) and SNAC (OR 3.13, 95% CI: 0.97-10.09, p = 0.056). Quantitative analysis for 5-year DFS was possible only for ONB, confirming a strong association between negative margins and better survival (OR 8.85, 95% CI: 3.55-22.11, p < 0.001). Details of histology-specific meta-analysis are reported in Figure 3.
Funnel plot analysis and Egger’s test (Supplementary Figure 1) revealed potential publication bias in the 5-year OS analysis across all studies (p = 0.008), as well as in the SNMM (p = 0.031), and ONB (p = 0.031) subgroups. In contrast, no significant asymmetry was found for 5-year DSS (p = 0.712), DFS (p = 0.423), or for SCC (p = 0.113), ACC (p = 0.275), ONB (p = 0.559), and SNAC (p = 0.974).
Discussion
Impact of surgical margins on survival
The results of this systematic review and meta-analysis confirm the significant prognostic role of surgical margins in sinonasal malignancies. Across all studies included, positive margins were associated with poorer survival outcomes, reinforcing the importance of achieving negative margins whenever feasible. While not all studies demonstrated a significant association between margin status and survival, a consistent trend emerged, highlighting the negative prognostic impact of margin infiltration. More importantly, considering only studies eligible for quantitative analysis, the present analysis demonstrated that patients with R1 resections had a 2.61-fold increased risk of mortality (OS), a 5.89-fold higher risk of disease-related mortality (DSS), and a 4.4-fold higher risk of recurrence (DFS).
Our findings are consistent with evidence from other head and neck malignancies, where the fundamental oncologic principle of achieving complete tumour resection has been widely recognised as essential for improving survival outcomes 73. However, at present, the prognostic value of margin status in sinonasal cancer is far from being completely clarified 74, even though multiple studies have demonstrated a correlation between negative surgical margins and better recurrence rates and survival outcomes 3,4,23,40,76-79. The result of the present review seems to confirm this trend, even though the interpretation of them must be approached with caution due to the significant heterogeneity among studies. While the pooled analysis suggests a detrimental impact of positive margins on survival, statistical significance was reached in only 10 of 28 individual OS studies. This variability may reflect methodological differences among studies or inherent limitations of the present analysis, which will be further discussed below.
Overall margin infiltration rates
Regardless of histology, this study found a high overall rate of margin infiltration (33.2%), in line with other review of the literature 4, highlighting the technical challenges in achieving negative margins in sinonasal malignancies, irrespective of the surgical approach. Historically, one of the main criticisms of endoscopic approaches was their supposed inability to achieve en-bloc resection (Cover figure). However, no clear evidence supports the superiority of en-bloc over piecemeal resection in terms of oncologic outcomes. Even craniofacial resection, theoretically enabling en-bloc excision, has shown high rates of margin infiltration, with one of the largest multicentric study on open resections 79 reporting positive margins in 31.5% of 1,307 cases. While endoscopic resections were initially reserved for early-stage tumours, advancements in technology and surgical expertise have extended their indications to advanced malignancies. A recent multicentric retrospective study on endoscopically treated sinonasal malignancies 77 found an overall margin infiltration rate of 20.5%, with the highest rates observed in MSGC (43%), SNUC/SNEC (33.3%), and SNMM (32.2%). Similarly, Abdelmeguid et al. 80 reported that negative margins were achieved in 87.4% of sinonasal cancer patients treated at the MD Anderson Cancer Center, despite nearly 40% of patients being affected by T4a or T4b staged disease. Finally, a recent international collaborative study 82 on skull base malignancies further reinforced these findings, reporting an overall margin infiltration rate of 26%, with significant variations depending on the surgical approach. Specifically, positive margins were identified in 32% of open resections, 25% of combined approaches, and only 18% of purely endoscopic procedures. These data suggest that, while the open approach is often reserved for more advanced tumours, the endoscopic technique can achieve comparable, if not superior, margin status in appropriately selected cases.
However, it is important to emphasise that such margin infiltration rates remain higher than those observed in other head and neck sites 73, underscoring the inherent challenges of sinonasal malignancies, irrespective of the surgical approach used for resection 4. Addressing these limitations should be a key focus of future efforts aimed at improving oncologic outcomes.
Histology-specific considerations: differences in the prognostic impact and rates of margin infiltration
Recent literature has demonstrated the association between the negative prognostic effect of margin involvement and the specific histology considered 1,22,4,77. The present review also highlighted differences in the prognostic impact of surgical margins among different histologic types, reinforcing the role of histology as a key determinant of sinonasal cancer prognosis and treatment selection.
ONB, SCC, and SNMM showed the strongest associations between negative margins and improved OS, suggesting that complete tumour resection is particularly crucial in these histotypes. This aligns with existing literature for SCC and ONB. For ONB, infiltrated postoperative margins are among the most recognised negative prognostic factors, along with advanced Kadish stage, high Hyams grade, and nodal metastases 26,38. Moreover, for ONB negative margin resection is also associated with a decreased risk of delayed recurrence in the neck, which has been demonstrated to be a significant predictor of mortality despite subsequent therapy 3,49. Similarly, the critical role of achieving negative margins in SCC is well-documented in the literature 76. Notably, the NCDB study by Jafari et al. 30 found that macroscopically involved margins were associated with survival outcomes comparable to those of patients treated with upfront non-surgical strategies, underscoring the importance of meticulous surgical planning to achieve at least macroscopically negative margins. Torabi et al. 78 further reinforced the prognostic value of negative margin resection in an NCDB review of 2,968 surgically treated patients with sinonasal SCC, where positive margins were associated with decreased OS (hazard ratio [HR] 1.672, 95% CI: 1.464-1.908) and a higher likelihood of requiring additional therapy (OR 1.966, 95% CI: 1.597-2.421). The likelihood of positive margins was higher in cases with advanced T-category (T4a vs T1), ethmoid sinus localisation (vs nasal cavity), and treatment at lower-volume centres, whereas the surgical approach (endoscopic vs. open) did not significantly impact margin status.
The prognostic impact of margin infiltration and OS for SNMM observed in our meta-analysis contrasts with some of the literature regarding this specific histology. In the multicentric experience of the European group reported by Ferrari et al. 22, SNMM was the only histology where the impact of negative margin resection did not show a statistically significant association with survival. This discrepancy may be attributed to the influence of specific studies in the pooled analysis, particularly Elsamna et al. 21, which reported an exceptionally strong prognostic impact of surgical margins. Additionally, the high heterogeneity (I2 = 66%) and the presence of small-study effects suggested by the funnel plot and Egger’s test (p = 0.031) indicate potential variability in patient populations, tumour characteristics, or treatment strategies across studies. Further investigation is needed to better define the prognostic role of infiltrated margins in SNMM 4, as well as the impact of surgery in the evolving therapeutic landscape, particularly in light of the emerging role of immunotherapy 82,83 and adjuvant proton beam therapy 84.
Of note, the present meta-analysis found no statistically significant impact of negative margin resection on OS for ACC and SNAC. In SNAC, the lack of statistical significance in our meta-analysis may be attributed to the limited sample size and the high heterogeneity among included studies (I2 = 84%), reducing the power to detect a significant association. Nonetheless, the observed trend (p = 0.056) suggests that the impact of negative margins on OS may become more evident with larger and more homogeneous datasets, as emerged in the recent consensus paper on sinonasal malignancies 4. However, in ACC, the lack of statistical significance may stem from the inherent challenges in achieving clear margins. ACC is characterised by perineural invasion and widespread microscopic submucosal tumour dissemination, making complete resection challenging or unfeasible, with reported R+ rates ranging from 63% to 85% 3,75,85,86. Moreover, even when surgical margins are reported as negative, skip lesions along nerve pathways may compromise the reliability of a truly margin-negative resection 87,88. The prognostic impact of positive margins in ACC remains therefore controversial 4. Some studies have reported a detrimental effect on survival, while others have not identified a significant association. Michel et al. 89 and Amit et al. 90 observed that positive margins were linked to lower 5-year OS and DFS, while Thompson et al. 91 did not find margin status to be an independent risk factor for DSS and DFS (p = 0.128). Wiseman et al. 92 noted a higher local recurrence rate among patients with positive margins compared to those with negative margins (41.7% vs 22.2%, p = 0.34), and further highlighted that local recurrence itself was a significant negative prognostic factor for 5-year OS (p = 0.05). The present review reinforces these controversial reports, highlighting ACC as the histology with the highest margin infiltration rate (61.5%). To note, a similarly high rate of margin infiltration was also observed in SNAC (42.9%) and SNUC (43.4%), suggesting that margin involvement is a critical issue not only in ACC but also in other aggressive sinonasal malignancies. For SNUC, the high rate of margin infiltration likely reflects both its biological aggressiveness and historical challenges in classification. Over the years, the diagnostic criteria for SNUC have evolved, and this category may have encompassed various poorly differentiated sinonasal carcinomas with distinct biological behaviours. Such undifferentiated tumours often present as high-stage lesions and surgical resection might not infrequently fail to achieve true negative margins. Multimodal treatment strategies – such as induction chemotherapy followed by chemoradiation or surgery based on treatment response – have become the standard of care. The fact that, historically, some cases may have been misclassified and treated primarily with surgery could partly explain the high rate of margin infiltration in this histology 93.
The high margin infiltration rate in SNAC (42.9%) is more complex to interpret, as multiple factors may have contributed to this finding. Some studies such as König et al.36, Li et al. 42, Zhou et al. 72 involved small patient cohorts, potentially inflating the infiltration rate due to selection bias. However, Vermassen et al. 69, who analysed a larger cohort, also reported a high rate of positive margins, though their series included a significant proportion of high-stage tumours treated with open approaches, which may have influenced the results. In contrast, Vedula et al. 68, in another large cohort study, reported a lower margin infiltration rate (28.2%), aligning more closely with large series on SNAC 2,77,94. This suggests that margin infiltration in SNAC may vary significantly depending on cohort size, tumour stage distribution, and surgical approach.
Heterogeneity in margin definition and assessment: the need for standardisation
The results of this review underscore that margin control remains a significant challenge, not only in terms of infiltration rates, as discussed previously, but also due to the substantial variability in how margins are defined, assessed, and managed across studies.
The distinction between R1 (microscopic infiltration) and R2 (macroscopic infiltration) was explicitly reported in only 8 studies, and also close margins were formally defined in just 8. Notably, in survival analyses, close margins were inconsistently classified, being grouped with either R0 (negative margins) or R+ (positive margins) across different studies, further complicating the interpretation of their prognostic impact. The absence of standardised classification not only limits the ability to compare results across studies, but also raises uncertainties about the actual prognostic relevance of close margins. Similarly, while some studies defined margins using a threshold distance in millimetres, this was a minority finding among the reviewed literature. To date, there is no robust evidence supporting a precise definition of an adequate resection margin. A strict distance threshold, as well as the principle of a close margin – concepts often translated from head and neck surgical oncology – may not be directly applicable to this anatomical region. The application of a millimetre-based cutoff may be feasible in open en-bloc resections, but its clinical utility is limited in endoscopic surgery, where margin assessment is influenced by visualisation, instrumentation, and anatomical constraints.4
One of the studies included deserves particular mention as it is the one that most clearly defined margin assessment. Ferrari et al. 22 explicitly reported that margins were assessed according to the multi-bloc technique, in which resection is performed using an oriented disassembly approach. This method preserves the spatial orientation of the removed structures, enabling a three-dimensional reconstruction of the margin status. In this regard, some teams have proposed the use of anatomical diagrams to precisely document resection margins and facilitate future multidisciplinary discussions on tumour management 95. However, no standardised approach has been established, and each institution currently manages this critical aspect of intraoperative margin assessment independently. This lack of standardisation inevitably introduces bias in the interpretation of surgical outcomes.
The concept of a layered margin, as described by Castelnuovo et al. 2 where margin negativity is determined by the deepest layer of the resection, is likely the most commonly technique employed today. This approach relies on direct intraoperative visualisation of tumour extension combined with an assessment of the presence or absence of infiltration. In recent years, additional intraoperative tools have shown promising results in optimising margin assessment. For example, neuro-navigation has demonstrated improved margin status in advanced malignancies of the anterior craniofacial region 96. Moreover, fluorescence-guided surgery has emerged as a promising technique to better delineate tissue invasion and assist in defining surgical margins through enhanced visualisation 97.
Frozen section analysis remains a fundamental intraoperative tool, offering real-time guidance on the need for additional resection. Given the potential for submucosal, subperiosteal, and perineural spread in sinonasal malignancies, gross tumour identification alone is insufficient to define resection margins, reinforcing the rationale for intraoperative margin assessment. However, while frozen sections are widely employed in sinonasal tumour surgery, their use was explicitly reported in only 7 studies included in this review, suggesting a potential underreporting. Although frozen section analysis has limitations – particularly the potential for misinterpretation of microscopic infiltration in intraoperative analysis, which is especially relevant in certain challenging histotypes such as SNMM 61,98 or in case of perineural invasion in ACC 86 – it remains a crucial tool for surgical decision-making and optimising oncologic outcomes in most of the cases as stated in a recent international consensus 4.
This variability in margin assessment underscores an important limitation when interpreting the prognostic impact of surgical margins. Without a standardised definition, comparing rates of recurrence and survival across studies becomes challenging, as different criteria may lead to significant discrepancies in margin status classification. More importantly, such a variability has consequences in clinical practice, where different margins definition might translate in differences in indication for adjuvant treatment. These findings emphasise the need for a more consistent, evidence-based approach to definition of surgical margins in sinonasal malignancies, which could ultimately improve cross-study comparisons and enhance prognostic accuracy.
Limitations of the study
This systematic review and meta-analysis provide valuable insights into the prognostic impact of surgical margins in sinonasal malignancies. However, several limitations must be considered when interpreting their findings.
First, the retrospective nature of all studies included, some of which are based on national cancer databases, introduces potential selection and reporting biases. Furthermore, evidence of publication bias – particularly in OS analyses and in SNMM and ONB subgroups – suggests that smaller studies with non-significant results may be underrepresented.
Significant heterogeneity was observed across studies and within specific subgroups (ONB, SCC, and SNAC), likely reflecting differences in study design, patient populations, histotypes, surgical approaches, and (neo-)adjuvant treatments. This variability is further compounded by the rarity and biological diversity of sinonasal malignancies, making large, homogeneous cohorts difficult to assemble. Consequently, most published literature consists of single-centre studies with relatively small sample sizes and short follow-up periods. The inclusion of studies spanning several decades (1956-2022) further adds clinical variability, as major advancements in diagnostic classification, staging, and treatment protocols have occurred over time. Importantly, the lack of standardised margin definitions and reporting criteria further complicates direct comparisons across different cohorts.
Despite the pivotal role of surgery in management of sinonasal tumours, oncologic outcomes are influenced by multiple factors, including histology, grade, stage, anatomical location, and the feasibility of (neo-)adjuvant therapy. Given that most included studies lacked sufficient outcome stratification based on these prognosticators, this review could not fully assess their interplay with margin status. This limitation restricts detailed subgroup analyses and control for confounders, underscoring the need for standardised reporting and more granular stratification in future studies.
Oncological outcomes were assessed at the 5-year timepoint, with only a few studies providing longer follow-up data, despite the extended patient recruitment period. The limited availability of long-term data prevented further analysis beyond this timeframe, which may be particularly relevant for histotypes such as ACC and ONB, where late recurrences are not uncommon.
Finally, while this meta-analysis focused on OS, DSS, and DFS, it did not assess recurrence patterns or the impact of margin status on local, regional, and distant recurrence. These factors were beyond the scope of the study and could not be thoroughly addressed. Future research should explore these aspects, as margin status may significantly influence recurrence patterns, which, in turn, are critical for long-term prognosis and treatment decisions.
Conclusions
This systematic review and meta-analysis confirms the prognostic significance of surgical margins in sinonasal malignancies. While not all studies demonstrated a significant association between margin status and survival, a clear trend emerged, highlighting the negative impact of resection within infiltrated margins.
The prognostic relevance of surgical margins, as well as the rate of margin infiltration, is strongly influenced by tumour histology, reaffirming its critical role in the management of sinonasal cancers. The high rate of margin infiltration observed across studies underscores the inherent challenges of surgical treatment in this anatomical region, driven by both tumour biology and complex local anatomy. Moreover, significant heterogeneity persists in the definition and management of surgical margins, limiting comparability across studies and complicating the interpretation of oncologic outcomes. These findings emphasise the urgent need for standardised surgical protocols and margin assessment criteria to enhance consistency in clinical practice and facilitate meaningful comparisons in future research.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
ADA, EC: contributed equally to this work and share first authorship; ADA, EC, MB: conceptualization; ADA, EC, MF, PG, AD, PN: methodology; ADA, EC, AL, GS, GD, GM: data curation, literature search, study selection, data extraction; ADA, EC, PG, MF, AD: statistical analysis; MF, PG, AD, PN, MB, ADA, EC: validation, writing and original draft preparation; ADA, EC, GM, GS, AL, GD, MF, PG, AD, PN, MB: writing, review, and editing; ADA, EC, MB, PC: visualization; MB, PC: supervision; PN, PC, MB: project administration. All authors have read and agreed to the published version of the manuscript.
Ethical consideration
Not applicable.
History
Received: March 12, 2025
Accepted: March 31, 2025
Figures and tables
Figure 1. PRISMA flow diagram illustrating the study selection process. The diagram details the number of records identified, screened, excluded, and included in the qualitative and quantitative analyses, following PRISMA guidelines.
Figure 2. Results of the meta-analysis evaluating the impact of surgical margin status on 5-year OS, DSS, and DFS across 30 studies on sinonasal malignancies. (A) Forest plot for 5-year OS, displaying individual study ORs with 95% CIs, along with the pooled effect estimate (OR = 2.61, 95% CI: 1.98-3.44, p < 0.001); (B) Funnel plot for 5-year OS, assessing potential publication bias (Egger’s test, p = 0.008), suggesting possible small-study effects; (C) Forest plot for 5-year DSS, showing the pooled OR (5.89, 95% CI: 4.22-8.22, p < 0.001), with low heterogeneity (I2 = 0%, p = 0.64); (D) Funnel plot for 5-year DSS, indicating no significant asymmetry (Egger’s test, p=0.712). (E) Forest plot for 5-year DFS, showing a pooled OR of 4.4 (95% CI: 2.12-9.11, p < 0.001), with moderate heterogeneity (I2 = 47%, p = 0.09); (F) Funnel plot for 5-year DFS, revealing no strong evidence of publication bias (Egger’s test, p = 0.423).
Figure 3. Results of the meta-analysis assessing the impact of surgical margin status on 5-year OS across different histological subgroups and 5-year DFS for ONB. Each forest plot presents individual study ORs with 95% CIs, along with the pooled effect estimate for each histology. Heterogeneity across studies was quantified using the I2 statistic and Cochran’s Q test. (A) Sinonasal mucosal melanoma (SNMM): OR = 4.89 (95% CI: 1.41-17, p = 0.013); (B) Squamous cell carcinoma (SCC): OR = 3.77 (95% CI: 1.52-9.4, p = 0.004); (C) Sinonasal adenocarcinoma (SNAC): OR = 3.13 (95% CI: 0.97-10.09, p = 0.056); (D) Adenoid cystic carcinoma (ACC): OR = 2.43 (95% CI: 0.53-11.19, p = 0.253); (E) Olfactory neuroblastoma (ONB): OR = 6.85 (95% CI: 2.6-18.09, p < 0.001); (F) Olfactory neuroblastoma (ONB) for DFS: OR = 8.85 (95% CI: 3.55-22.11, p < 0.001).
Supplementary Figure 1. Funnel plots of the meta-analysis on 5-year OS across different histological subgroups (SNMM, SCC, SNAC, ACC, ONB) and 5-year DFS for ONB. Egger’s test p values are reported for publication bias assessment.
| Study | Year | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal 9 | 2023 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Fair |
| Al-Qurayshi 10 | 2021 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Amit 11 | 2017 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Fair |
| Auger 12 | 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Bahig 13 | 2023 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Bristol 14 | 2007 | Yes | Yes | CD | Yes | Yes | Yes | No | No | Yes | Fair |
| Chao 15 | 2001 | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Choby 16 | 2023 | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Cracchiolo 17 | 2018 | Yes | Yes | CD | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Crawford 18 | 2020 | Yes | Yes | CD | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Duru Birgi 19 | 2024 | Yes | Yes | CD | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Eide 20 | 2023 | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Elsamna 21 | 2021 | Yes | Yes | CD | Yes | No | Yes | Yes | Yes | Yes | Fair |
| Ferrari 22 | 2022 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Ganti 23 | 2019 | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Guazzo 24 | 2019 | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Guo 25 | 2022 | Yes | Yes | CD | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Harvey 26 | 2016 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Herr 27 | 2013 | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Hirakawa 28 | 2016 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Issa 29 | 2023 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Jafari 30 | 2019 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Khan 31 | 2017 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Kilic 32 | 2017 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Kim 33 | 1999 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Konig 34 | 2017 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Konig 35 | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Konig 36 | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Konuthula 37 | 2016 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Konuthula 38 | 2017 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Lehrich 39 | 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Li 40 | 2020 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Li 41 | 2021 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Li 42 | 2023 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Lian 43 | 2024 | Yes | Yes | No | CD | Yes | Yes | No | Yes | No | Fair |
| Malouf 44 | 2012 | Yes | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Good |
| McMillan 45 | 2022 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Nakagawa 46 | 2017 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Nakamaru 47 | 2020 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Nakaya 48 | 2004 | Yes | Yes | No | CD | Yes | Yes | No | Yes | No | Fair |
| Nalavenkata 49 | 2015 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Nishio 50 | 2015 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Ogawa 51 | 2000 | Yes | Yes | No | CD | Yes | Yes | Yes | Yes | Yes | Fair |
| Ono 52 | 2018 | Yes | Yes | No | CD | Yes | Yes | Yes | Yes | Yes | Fair |
| Paré 53 | 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Parikh 54 | 2021 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Patel 55 | 2012 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Patel 56 | 2024 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Petruzzelli 57 | 2015 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Qatanani 58 | 2023 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Resto 59 | 2000 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Rojas Lechuga 60 | 2022 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Sayed 61 | 2017 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Sun 62 | 2020 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Tajudeen63 | 2014 | Yes | Yes | No | CD | Yes | Yes | No | Yes | Yes | Fair |
| Torabi64 | 2019 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Tsutsumi 65 | 2023 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Tsutsumi 66 | 2024 | Yes | Yes | Yes | CD | Yes | Yes | No | Yes | Yes | Fair |
| Ungar 67 | 2024 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Vedula 68 | 2023 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Vermassen 69 | 2024 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Wang 70 | 2024 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Good |
| Zafereo 71 | 2008 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Zhou 72 | 2024 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Fair |
| Quality assessment of the included studies according to the National Heart, Lung and Blood Institute (NHLBI), Study Quality Assessment Tools: C1, “Was the study question or objective clearly stated?”; C2: “Was the study population clearly and fully described, including a case definition?”; C3: “Were the cases consecutive?”; C4: “Were the subjects comparable?”; C5: “Was the intervention clearly described?”; C6: “Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants?”;C7: “Was the length of follow-up adequate?”; C8: “Were the statistical methods well-described?”; C9: “Were the results well-described?”. | |||||||||||
| CD: cannot determine. | |||||||||||
| First author (year) | Study | Demographics | Tumour | Treatment | Margin status | Follow-up | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Age (years) mean or #median (range, IQR or SD) | Gender (M/F) | Histology | Initial location | T Stage | N. Stage | Surgery (approach) | Previous treatment | Adjuvant treatment | R0/R+/R close/unknown | Rate of positive margins (%) | F.U. (months)mean or #median (range, IQR or SD) | Investigated outcome (model) | association (* significant) | |||
| Agarwal 2023 9 | RCS(USA 2012-2018) | 50 | #65.6(IQR: 52.8-74.4) | 32/18 | SCC: 50 | NC: 25MS: 17ES: 2FS: 1NR: 5 | Tis-T2:14T3: 27unknown: 9 | N0: 28N+: 9NR: 13 | 47(EEA: 25Open: 22) | NR | RT: 26RT-CHT: 7 | R0: 34R+: 8unknown: 5 | 19 | NR | DFS (u) | HR R+ 6.76 (CI 1.35-34.0), p = 0.020*DFS (m) | HR R+ 3.10 (CI 0.39-24.6), p = 0.285 | |
| Al-Qurayshi 2021 10 | NCDB(USA 2004-2015) | 574 | 61.7(SD: 16.5) | 320/254 | SNAC: 213ACC: 131SNMM: 115ONB: 68SNUC: 47 | NR | NR | NR | 574(NR) | RT: < 10°CHT: < 10°RT-CHT: 41 | NR | R0: 311R+: 263 | 45.8 | #40.4(IQR: 15.3-81.3) | OS (m) | HR R0 0.55 (CI 0.36-0.82), p = 0.004* | |
| Amit 2017 11 | RCS(USA 1991-2016) | 66 | 63(34-85) | 33/33 | SNMM: 66 | NC: 53MS: 8SS: 3ES: 1FS: 1 | T3: 35T4a: 23T4b: 8 | N0: 59N+: 7 | 66(NR) | NR | RT: 30RT-CHT: 6 | R0: 44R+: 22 | 33.3 | 40.1(SD 5.6) | OS (u) | p = 0.009*OS (m) | HR R+ 5.43 (CI 1.44–21.85), p = 0.01*DSS (m) | HR R+ 21.9 (CI 3.71–180), p = 0.0004* | |
| Auger 2020 12 | NCDB(USA 2004-2015) | 239 | < 50y: 5650-60y: 6260-70y: 60> 70y: 61 | 125/114 | MEC: 239 | MS: 109NC: 98SS: 5FS: 3ES: 1other: 10 | NR | NR | 198(NR) | RT: 185; CHT: 38 (before or after surgery) | R0: 114R+: 55unknown: 29 | 32.5 | NR | OS (u) | p = 0.048* | ||
| Bahig 2023 13 | RCS(USA 2000-2016) | 311 | #57(20-90) | 193/118 | SCC: 132SNUC: 45ACC: 45ONB: 41SNEC: 24SNAC: 17MEC: 3other: 4 | NC: 132MS: 102ES: 47FS: 17SS: 4other: 9 | T1: 15T2: 38T3: 39T4: 219 | N0: 263N+: 48 | 211(EEA: 91Open: 120) | NR | 211 | R0: 170R+: 41 | 19.4 | #75(1-186) | OS (u) | RR R+ 1.54 (CI 0.96-2.49), p = 0.07PFS (u) | RR R+ 1.49 (CI 1-2.22), p = 0.05OS (m) | RR R+ 1.54 (CI 0.96 2.49), p = 0.02* | |
| Bristol 2007 14 | RCS(USA 1969-2002) | 146 | #59(26-90) | 86/60 | SCC: 89ACC: 33SNAC: 6SNUC: 11other: 7 | MS: 146 | T1-T2: 22T3: 47T4: 77 | N0:126N+: 20 | 146(NR) | CHT: 24 | RT: 146 | R0: 95R+: 37(R1: 33; R2: 4)R close: 12unknown: 2 | 25.7 | #46(4-357) | OS (m) | HR R0 0.636 (CI 0.407-0.994), p = 0.047* | |
| Chao 2001 15 | RCS(USA 1976-1996) | 25 | #57(16-73) | 11/14 | ONB: 25 | NR | Kadish A: 3Kadish B: 13Kadish C: 8Kadish D: 1 | NR | 19(Open: 19) | NR | RT: 9RT-CHT: 8 | R0: 10R+: 5unknown: 4 | 33.3 | #96(24-288) | DFS (u) | p > 0.05 | |
| Choby 2023 16 | RCS(USA 2005-2021) | 256 | 52(43-63) | 141/115 | ONB: 256 | NR | Kadish A: 26Kadish B: 52Kadish C: 136Kadish D: 34NR: 8 | NR | 243NR: 2(EEA: 161Open: 82) | CHT: 12 | RT: 137RT-CHT: 57 | R0: 154R+: 60unknown: 31 | 28 | NR | OS(u) | HR R+ 2.09 (CI 1.13, 3.87)*OS(m) | HR R+ 3.78 (CI 1.82, 7.88)*DFS | HR R+ 2.06 (CI 1.22, 3.48)*DSS | HR R+ 2.25 (CI 0.71, 7.11) | |
| Cracchiolo 2018 17 | NCDB(USA 2003-2012) | 4770 | < 50y: 58350-64y: 163765-79y: 1775> 80y: 775 | 3041/1729 | SCC: 4770 | NC: 2512MS: 1961ES: 297 | T1: 1474T2: 743T3: 762T4a: 1197T4b: 594 | N0: 4478N+: 292 | 1717(NR) | NR | RT: 670RT-CHT: 395 | R0: 1212R+: 475(R1: 273; R2: 202)unknown: 30 | 28.2 | 55(0-128.5) | OS (u) | p < 0.001*OS (m) | R1 OR 1.74 (CI 1.4-2.16) p < 0.001*R2 OR 1.43 (CI 1.14-1.8) p = 0.002* | |
| Crawofrd 2020 18 | NCDB(USA 2004-2016) | 1120 | 63.9(NR) | 747/373 | SCC: 1120 | MS: 589NC: 421ES: 110 | T3-T4: 1120 | N0: 1089N+: 28NR: 3 | 1120(NR) | NR | RT: 861CHT: 373 | R0: 376R+: 618unknown: 126 | 62.2 | #54.5(NR) | OS (u) | p = 0.012*OS (m) | HR R+ 1.309 (CI 1.078-1.590), p = 0.007* | |
| Duru Birgi 2024 19 | RCS(Turkey 2001-2021) | 194 | #58(23-92) | 136/58 | SCC: 104ACC: 36SNAC: 14other: 40 | NC: 64MS: 11ES: 10 | T1: 1T2: 4T3: 61T4a: 100T4b: 28 | N0: 165N+: 29 | 155(NR) | NR | RT: 155 | R0: 67R+: 74R close: 6unknown: 8 | 50.3 | 43(6-158) | OS, PFS, LRFS, DMFS (u) | p > 0.05DMFS (m) | HR R+ 4.409 (CI 1.369-14.19), p = 0.013* | |
| Eide 2023 20 | NCDB(USA 2004-2016) | 38 | 60.7(SD:18.1) | 18/20 | MEC: 38 | NC: 19MS: 16ES: 2other: 1 | NR | NR | 35(NR) | RT:16; CHT: 4 (before or after surgery) | R0: 21R+: 6unknown: 8 | 22.2 | NR | OS (u) | p > 0.05 | ||
| Elsamna 2021 21 | NCDB(USA 2010-2015) | 446 | ≤ 65y: 146≥ 66y: 300 | 204/242 | SNMM: 446 | NC: 364PNS: 82 | T3: 244T4: 163NR: 39 | N0: 403N+: 24NR: 19 | 383(EEA: 90Open: 160NR: 133) | NR | RT: 207 | R0: 263R+: 120 | 31.3 | NR | OS (u) | p < 0.001* | |
| Ferrari 2022 22 | RCS(Europe 1995-2021) | 1360[selected histology: 824] | 62(8-89) | 574/250 | SNAC: [348]SNC: [144]ONB: [112]SNMM: [87]SNUC: [54]MSGT: [79] | NC + ES: [741]MS: [62]SS: [11]FS: [4]NR: [6] | T1: [129]T2: [172]T3: [160] | N0: [804]N+: [20] | 824(EEA: [733]Open: [91]) | NR | RT/RT-CHT: [535] | R0: [646]R+: [178(R1:156; R2:22)] | 21.6 | [58 (1-213)] | SNAC (m) | OS*, CSS*, RFS*, LRFS*, RRFS*, DRFS*SCC (m) | OS*, CSS*, RFS*, LRFS*, RRFS, DRFSONB (m) | OS, CSS, RFS*, LRFS*, RRFS, DRFSMM (m) | OS, CSS, RFS, LRFS, RRFS, DRFSSalivary (m) | OS, CSS*, RFS, LRFS, RRFS, DRFS | |
| Ganti 2019 23 | NCDB(USA 2004-2015) | 1874 | 71(NR) | 1000/874 | SNMM: 1874 | NC: 1312MS: 252ES: 114FS: 7SS: 4other: 146 | T3: 442T4: 324NR: 1126 | N0: 718N+: 65NR: 1091 | 1167(EEA: 307 Open: 390NR: 470) | RT: 1093; CHT: 223;IT: 158 (before or after surgery) | R0: 812R+: 355 | 30.4 | NR | OS (u) | p < 0.001*OS (m) | HR R0 0.44 (CI 0.30-0.65) < 0.001* | ||
| Guazzo 2019 24 | RCS(Australia 2005-2017) | 32[sinonasal: 17] | 45.2(26-73) | 8/9 | [ACC: 17] | NR | T3-T4b: [17] | NR | [17](EEA: [7]Open: [10]) | NR | [RT: 17] | R0: [2]R+: [15] | 88.2 | #82.1(33.1-159.5) | NR | |
| Guo 2022 25 | RCS(USA NR) | 45 | #71.8(41.6-90.9) | 17/28 | SNMM: 45 | NR | T3: 25T4a: 11T4b: 9 | NR | 45(NR) | NR | RT: 43CHT: 21ICIs: 15 | R0: 31R+: 5unknown: 9 | 13.9 | 38.7(6.2-287) | OS (u) | HR R+ 1.96 (0.56, 5.43), p = 0.26RFS (u) | HR R+ 1.58 (0.53, 3.88), p = 0.38 | |
| Harvey 2016 26 | RCS(Australia NR) | 109 | 49.2(NR) | 59/50 | ONB: 109 | NR | Kadish A: 11Kadish B: 27Kadish C: 71Kadish D: 1 | NR | 109(EEA: 67Open: 42) | RT: 9 | RT: 81 | R0: 81R+: 28 | 25.7 | 42(6-421) | OS (u), p = 0.004* | |
| Herr 2013 27 | RCS(USA 1997-2013) | 22 | #45.5(11-77) | 11/11 | ONB: 22 | NR | Kadish B: 10Kadish C: 12 | N0: 19N+: 3 | 22(Open: 22) | CHT: 3 | IMPT: 17 IMPT-CHT: 5 | R0: 13R+: 9 | 40.9 | 73(NR) | OS (u) p = 0.269DFS (u) p = 0.015* | |
| Hirakawa 2016 28 | RCS(Japan 2000-2009) | 58 | 60(35-77) | 51/7 | SCC: 58 | MS: 53ES: 5 | T2: 3T3: 14T4: 41 | N0: 46N+: 12 | 58(NR) | CHT: 43RT: 11 | RT: 11 | R0: 48R+: 10 | 17.2 | #40(1-156) | LRC (u) | HR R+ 5.89 (1.92-17.14), p = 0.03*DMFS (u) | HR R+ 8.89 (2.96-26.84) 0.02*DFS (u) | HR R+ 8.63 (3.32-22.4) < 0.0001* | |
| Issa 2023 29 | NCDB(USA 2004-2014) | 1883 | 64.7(20-90) | 1233/650 | SCC: 1883 | NR | T1: 807T2: 410T3: 214T4: 452 | N0: 1673N+: 210 | 994(NR) | CHT: 9 | RT: 408RT-CHT: 117 | R0: 781R+: 213 | 21.4 | NR | OS (m) | HR R+ 1.48 (CI 1.06-2.07); p = 0.02* | |
| Jafari 2019 3 | NCDB(USA 2004-2015) | 7808 | 64.8(SD:13.2) | 5059/2749 | SCC: 7808 | PNS: 4298NC: 3510 | T1: 1255T2: 946T3: 1064T4: 2640NR: 1903 | N0: 4824N+: 1104NR: 1880 | 3322(NR) | 166 | 2892 | R0: 2289R+: 1033(R1: 511; R2: 521) | 31.1 | NR | OS HR R0 vs non-surg 0.54, p < 0.0001*OS HR R1 vs non-surg 0.78, p < 0.0001*OS HR R2 vs non-surg 0.92, p > 0.05 | |
| Khan 2017 31 | NCDB(USA 2004-2010) | 460[late stage: 277] | 55.8(SD:15.3) | 173/104 | SNUC: 277 | NR | NR | NR | [96](NR) | NR | RT-CHT: [96] | R0: [37]R+: [22]unknown: [37] | 37.3 | NR | NR | |
| Kilic 2017 32 | NCDB(USA 2010-2014) | 1438 | < 60y: 52460-70y: 475> 70y: 484 | 963/520 | SCC: 1438 | NC: 855MS: 418ES: 104SS: 27FS: 21other: 58 | NR | N0: 1438N+: 0 | 1438(EEA: 353 Open: 1130) | 0 | RT/RT-CHT: 772 | R0: 918R+: 341unknown: 179 | 27.1 | NR | OS (u) | HR R+ 1.72 (CI 1.39-2-13), p < 0.001*OS (m) | HR R+ 1.51 (CI 1.19-1.91), p = 0.001* | |
| Kim 1999 33 | RCS(Korea 1975-1993) | 22 | 48(26-68) | 16/6 | ACC:22 | NR | T2: 4T3: 9T4a: 9 | N0: 22 | 13(Open: 13) | NR | RT: 10 | R0: 5R+: 8 | 61.5 | 96(60-168) | p = 0.98 | |
| Konig 2017 34 | RCS(Norway 1998-2016) | 20 | 58.1(42-76) | 12/8 | ONB: 20 | NR | T1: 3T2: 8T3: 6T4: 3 | NR | 17(Open: 17) | RT: 2 | RT: 11RT-CHT: 4 | R0: 10R+: 7 | 41.2 | 86.2(23.2-216) | p < 0.001* | |
| Konig 2019 35 | RCS(Norway 1988-2017) | 72 | 67.1(36-94) | 51/21 | SCC: 72 | MS: 60ES: 8FS: 4 | T1: 2T2: 4T3: 7T4a: 20T4b: 39 | N0: 58N+: 14 | 34(EEA: 1Open: 33) | RT: 5CHT: 1 | RT: 18RT-CHT: 5 | R0: 21R+: 13 | 38.2 | 57(1-314) | DSS (u) | p = 0.002*DSS (m) | p = 0.002* | |
| Konig 2019 36 | RCS(Norway 1995-2018) | 20 | 57.5(25-81) | 15/5 | SNAC: 20 (ITAC: 13; nITAC: 7) | ES: 17MS: 3 | T1: 2T2: 3T3: 4T4: 11 | N0: 20 | 18(EEA: 2Open: 16) | NR | RT: 2 | R0: 10R+: 8 | 44.4 | 89(1-239) | p = 0.005* | |
| Konuthula 2016 37 | NCDB(USA 2004-2010) | 695 | 68.9(SD: 13.4) | 316/379 | SNMM: 695 | NC: 470PNS: 225 | NR | NR | 555(NR) | 6 | RT: 271RT-CHT: 39 CHT: 29 | R0: 300R+: 127unknown: 268 | 29.7 | NR | OS (u) | p = 0.001*OS (m) | HR R0 0.74 (CI 0.56-0.97), p = 0.03* | |
| Konuthula 2017 38 | NCDB(USA 2004-2013) | 1167 | 54(18-90) | 706/461 | ONB: 1167 | NR | Kadish A: 173 Kadish B: 221 Kadish C: 621 Kadish D: 92NR: 60 | N0: 1015N+: 92NR: 60 | 989(NR) | 25 | RT: 404RT-CHT: 300 CHT: 17 | R0: 481R+: 215unknown: 293 | 30.9 | NR | OS (u) | p = 0.0005*OS (m) | HR R0 0.62 (CI 0.40-0.96), p = 0.0302* | |
| Lehrich 2020 39 | NCDB(USA 2004-2015) | 3011 [salvage surgery: 207] | 56.6(SD:13.6) | 140/67 | SCC: [125]ONB: [38]SNUC:[25]SNAC: [19] | NC: [90]MS: [82 ]ES: [25]FS: [2]SS: [2]other: [6] | NR | N0: [130]N+: [26]NR: [51] | [207](EEA: [16] Open: [74]NR: [117]) | CHT: [44]RT-CHT: [97] RT: [66] | NR | R0: 115R+: 54unknown: 38 | 32 | NR | OS (u) | HR R+ 1.84 (CI 1.13-3), p = 0.02*OS (m) | HR R+ 2.31 (CI 1.29-4.13), p = 0.005* | |
| Li 2023 40 | RCS(China 2000-2020) | 12 | 48(33-65) | 10/2 | SNAC (nITAC): 12 | NC: 11ES: 1MS: 2 | T1-T3: 6T4: 6 | N0-N1: 8N2: 4 | 11(EEA: 11) | NR | RT: 3RT-CHT: 5 TT: 1 | R0: 6R+: 5 | 45.5 | 47(17-106) | RR | p = 0.33DSS | p < 0.01* | |
| Li 2020 41 | RCS(China 2007-2017) | 21 | 59.2(35-81) | 18/3 | SCC:21 | MS: 11NC/other: 10 | T1: 2T2: 1T3: 10T4: 8 | NR | 21(EEA: 16Open: 5) | NR | RT: 7RT-CHT: 7 CHT: 2CHT + IT: 1 | R0: 15R+: 6 | 28.6 | 47.4(3-123) | OS (u) | p < 0.001OS (m) | HR R+ 1.931 (CI 1.082-3.447), p = 0.026* | |
| Li 2021 42 | RCS(China 2005-2018) | 173 | 54.3(NR) | 128/45 | SCC: 173 | MS: 118NC/ES: 41FS+SS: 14 | T2: 32T3: 63T4: 78 | N0: 168N+: 5 | 173(EEA: 51Open: 122) | NR | 153 | R0: 100R+: 73 | 42.2 | #65(6-157) | OS (u) | p = 0.186 | |
| Lian 2024 43 | RCS(China 2004-2021) | 219 | #54(16-82) | 155/64 | SCC: 219 | MS: 111NC: 66ES: 26other: 16 | T1: 12T2: 55T3: 77T4: 75 | N0: 199N+: 20 | 219(EEA: 88Open: 131) | NR | NR | R0: 131R+: 66R close: 11unknown: 11 | 31.7 | 43.9(2-164) | OS (u) | HR R0/close vs R+ 0.137(95% CI 0.0770.243), p < 0.0001*OS (u) HR R0 vs Rclose 0.210(95% CI 0.076-0.580), p < 0.01*RFS (u) | HR R0/close vs R+ 0.111(95 % CI 0.064-0.192), p < 0.0001*RFS (m) | HR R0 vs Rclose 0.263(95% CI 0.088-0.780), p < 0.01* | |
| Malouf 2012 44 | RCS(France 1979-2009) | 44 | #43(4-78) | 23/21 | ONB: 44 | NR | Kadish B: 16Kadish C: 15 | N0: 27N1: 4 | 31(NR) | CHT: 11 | RT: 29CHT: 6 | R0: 22R+: 9 | 29 | 11(1-356) | OS (u) | p = 0.002*DFS (u) | p < 0.001*OS (m) | HR R+ 5.94 (95% CI 1.23-28.77) p = 0.03*DFS (m) | HR R+ 7.09 (95% CI 2.1-23.89) p = 0.002* | |
| McMillan 2022 45 | RCS(USA 1960-2020) | 143 | #50(IQR 40-58) | 80/58 | ONB: 143 | NR | Kadish A: 6Kadish B: 26Kadish C: 79Kadish D: 16 | N0: 124N+: 19 | 135(EEA: 31Open: 104) | NR | RT: 111CHT: 66 | R0: 67R+: 30unknown: 38 | 30.9 | #76.6(IQR 25.5-153) | OS, DMFS (u) | n.s.PFS (u) | p < 0.05*OS (m) | HR R+ 1.67 (IC 0.78-3.58) p = 0.19PFS (m) | HR R+ 1.96 (IC 1.10-3.5) p = 0.02*DMFS (m) | HR R+ 0 (IC 0-0) p = 0.99 | |
| Nakagawa 2017 46 | RCS(Japan 2008-2016) | 22 | 49(27-83) | 10/12 | ONB: 22 | NR | Kadish A: 4Kadish B: 5Kadish: 13 | N0: 22 | 22(EEA: 22) | 0 | RT: 20 | R0: 21R+: 1 | 4.5 | 44(11-104) | NR | |
| Nakamaru 2020 47 | RCS(Japan 2005-2019) | 15 | #68(47-81) | 8/7 | SCC: 15 | NC: 6MS: 5ES: 4 | T1:4T2: 7T3: 4 | N0: 15N+: 0 | 15(EEA: 15) | 0 | RT: 5RT-CHT: 2 | R0: 11R+: 4 | 26.7 | #26(7-123) | OS (u) | p = 0.138DSS (u) | p = 0.0253 | |
| Nakaya 2004 48 | RCS(Japan 1980-2000) | 16 | 65.5(53-79) | 11/5 | SNMM: 16 | NC: 16 | NR | N0: 14N+: 2 | 14(Open: 14) | RT/RT-CHT:10 (before or after surgery) | R0: 7R+: 7 | 50 | 37(4-150) | n.s. | ||
| Nalavenkata 2015 49 | RCS(Australia-USA1986-2013) | 113 | 49.7(SD 13.2) | 61/52 | ONB: 113 | NR | Kadish A: 11Kadish B: 30Kadish C: 72 | N0: 115N+: 8 | 109(EEA: 67Open: 42) | RT: 10 | RT: 82 | R0: 80R+: 28unknown: 5 | 25.9 | 41.5(IQR 58.2) | RRFS (u) | R+ 17.9% vs 5%, p = 0.034* | |
| Nishio 2015 50 | RCS(Japan 1992-2011) | 40[survival analysis: 35] | 58.1(43-75) | 29/11 | SCC: 32ACC: 2SNAC: 2MEC: 1SNUC:1 other: 2 | MS: 40 | T4a: 26T4b: 14 | N0: 35N+: 5 | 40 [35](Open: 40) | RT: 6CHT: 10RT-CHT: 12 surgery + RT: 6 | NR | R0: [26]R+: [9] | 25.7 | #40(1-158) | OS (u) | p = 0.019* | |
| Ogawa 2000 51 | RCS(Japan 1979-1998) | 41 | #55(37-79) | 32/9 | SCC: 41 | MS: 41 | T2: 6T3: 22T4: 13 | N0: 36N+: 5 | 41(Open: 41) | CHT: 16 | RT: 41 | R0: 14R+: 27(R1: 23; R2: 4) | 65.9 | #93(25-179) | LCR (u) | p < 0.01* | |
| Ono 2018 52 | RCS(Japan 1998-2016) | 76[salvage surgery: 14] | 66(SD: 10) | 61/15 [12/2] | SCC: 70 [12]MEC: 2 [1]SNUC:1 [0] other: 3 [1] | NR | T2: 2 [1]T3: 24 [5]T4a: 44 [6]T4b: 6 [2] | N0: 67 [12]N+: 9 [2] | 29 [14](Open: 29 [14]) | RT-CHT: 29 [14] Surgery: 1 [1] | NR | R0: [7]R+: [7] | 50 | #60.9(14.1-147.1) | OS (u) | p = 0.004 LCR (u) | p = 0.005” | |
| Paré 2017 53 | RCS(France 1998-2012) | 68 | #63(17-88) | 43/25 | SCC: 68 | MS: 44NC: 11ES: 9other: 4 | T1: 2T2: 7T3: 14T4a: 39T4b: 6 | N0: 56N1: 4N2: 8 | 68(Open: 68) | Surgery: 6Surgery+RT: 5CHT-RT: 3CHT: 40 | RT: 39RT-CHT: 19 | R0: 36R+: 29unknown: 3 | 44.6 | #68(IQR: 39–112) | OS (u) | HR R+ 1.82 [0.87-3.79], p = 0.11LRFS (u) | HR R+ 1.75 [0.76-3.98], p = 0.19 | |
| Parikh 2021 54 | RCS(USA 2006-2013) | 48 | 65.8(19-95) | 31/17 | SCC: 48 | NR | T1-3: 18T4: 30 | N0: 41N+: 7 | 48(Open: 48) | CHT: 1 | RT: 17CHT: 21 | R0: 23R+: 25 | 52.1 | 40.7(NR) | OS (u) | RR R+ 0.9, p = 0.79DFS (u) | RR R+ 1.2, p = 0.74OS (m) | RR R+ 1.0, p = 0.99DFS (m) | RR R+ 1.4, p = 0.53 | |
| Patel 2012 55 | RCS(USA 1956-2000) | 151 | #49.5(12-82) | 88/58NR: 5 | ONB: 151 | NR | Kadish A/B: 33Kadish C: 116 | NR | 15(Open: 151) | Surgery: 50RT: 51CHT: 23 | RT: 71CHT: 9 | R0: 102R+: 23R close: 20unknown: 6 | 15.9 | #56(1-323) | OS (u) | p = 0.01*DSS (u) | p = 0.009*RFS (u) | p = 0.01*OS (m) | HR R+ 2.2 (IC 1.1-4.7), p = 0.02*DSS (m) | HR R+ 2.4 (IC 1.1-5.3), p = 0.04*RFS (m) | HR R+ 2.1 (IC 1.1-4.0), p = 0.01* | |
| Patel 2024 56 | NCDB(USA 2004-2019) | 1750 [surgery: 513] | #55(IQR: 45-65) | 312/201 | SNAC: [114] ACC: [111]SNUC:[107]SNEC: [71]ONB: [55]SNMM: [47]MEC: [8] | NC: [201]ES: [140]MS: [72]SS: [16]FS: [11]other: [73] | T4b: [513] | N0: [342]N+ : [51]NR: [120] | [513]([NR]) | [53] | [398] | R0: [174]R+: [102(R1: 69, R2:33,NR: 100)]unknown: [137] | 53.7 | NR | OS (u) | p = .059 | |
| Petruzzelli 2015 57 | RCS(USA 1994-2012) | 32 | 51(13-77) | 21/11 | ONB:32 | NR | Kadish A: 6Kadish B: 13Kadish C: 13 | N0: 30N+: 2 | 32(EEA: 9Open: 22NR: 1) | RT-CHT: 4 | RT: 19RT-CHT: 5 | R0: 25R+: 7 | 21.9 | 96.1(6-240) | NR | |
| Qatanani 2023 58 | NCDB(USA 2004-2016) | 173 | 56.6 | 114/59 | SNUC:173 | NR | T1: 9T2: 10T3: 14T4: 62NR: 76 | N0: 173 | 173(NR) | NR | RT: 34RT-CHT: 139 | R0: 56R+: 55unknown: 62 | 49.5 | NR | OS (u) | p < 0.05*OS (m) | HR R+ 4.82 (CI 2.28-10.2), p < 0.001* | |
| Resto 2000 59 | RCS(USA 1981-1998) | 27 | #52(17-77) | 14/13 | ONB: 27 | NR | T1: 11T2: 10T3: 6 | N0: 22N+: 5 | 17(Open: 17) | RT: 4CHT: 3 | RT: 9CHT: 1RT-CHT: 4 | R0: 10R+: 7unknown: 1 | 41.2 | 5.9(NR) | OS (u) | HR R+ NR (CI NR), p < 0.05*DFS (u) | HR R+ 10.1 (CI 1.1-95.9), p = 0.04* | |
| Rojas Lechuga 2022 60 | RCS(Spain 1984-2020) | 50 | 70.4(SD 12.5) | 24/26 | SNMM: 50 | PNS: 26NC: 24 | T3: 22T4a: 19T4b: 9 | N0: 43N1: 7 | 46(EEA: 22Open: 23) | NR | RT: 20 | R0: 15R+: 14unknown: 17 | 48.3 | #39.6(IQR 78.5) | DSS (u) | HR R+ 1.8 (CI 1.0-3.0), p = 0.045*DSS (m) | HR R+ 12.4 (CI 0.6-0.6), p = 0.231 | |
| Sayed 2017 61 | RCS(USA 1998-2016) | 72 | < 60y: 20≥ 60y: 52 | 33/39 | SNMM: 72 | NC: 50PNS: 22 | T3: 42T4: 30 | N0: 72N+: 0 | 72(EEA: 20Open: 52) | RT:58 (adjuvant)IT (before or after surgery) | R0: 40R+: 32 | 44.4 | NR | OS (m) | HR R+ 1.55 (CI 0.84-2.85)LRFS (m) | HR R+ 1.37 (0.66-2.88) | ||
| Sun 2020 62 | RCS(China 1984-2018) | 138 | #36(7-81) | 91/47 | ONB: 138 | NR | Kadish A: 1Kadish B: 25Kadish C: 112 | N+: 34 | 88(EEA: 45Open: 43) | RT: 33CHT-RT: 17 | RT: 49CHT-RT: 3 | R0: 38R+: 50 | 56.8 | #61(4-231) | NR | |
| Tajudeen 2014 63 | RCS(India 1991-2011) | 14 | 64(SD 12) | 7/7 | SNMM:14 | NC: 11MS: 2ES: 1 | T1: 6T2: 2T4a: 6 | NR | 14(NR) | Surgery: 2 Surgery + RT: 1 IFN+Surgery: 1 IFN: 1CHT: 1 | RT: 8RT-CHT: 2 | R0: 10R+: 4 | 28.6 | 20.7(1.4-84.5) | OS (u) | p = n.s.LRFS (u) | p = 0.031* | |
| Torabi 2019 64 | NCDB(USA 2004-2014) | 556 | 18-54y: 21455-64y: 12965-74y: 119≥ 75y: 94 | 273/283 | ACC: 403MEC: 89other: 64 | MS: 282NC: 239ES: 35 | T1: 130T2: 91T3: 176T4a: 159 | N0: 532N+: 24 | [506-514]°(NR) | 0 | RT: 293RT-CHT: 52 CHT: < 10° | R0: 223R+: 180unknown: [103-111]° | 44.7 | NR | p < 0.001* | |
| Tsutsumi 2023 65 | NCDB(USA 2004-2016) | 814 | 52.6(SD: 15.1) | 492/322 | ONB: 814 | NR | Kadish A-B: 287 Kadish C-D: 527 | NR | 814(NR) | 0 | RT: 562RT-CHT: 252 | R0: 631R+: 183 | 22.5 | NR | OS (m) | HR R+ 1.44 (CI 1.01-2.05), p = 0.04* | |
| Tsutsumi 2024 66 | RCS(USA 2014-2020) | 22 | 52(29-76) | 13/9 | ONB: 22 | NR | Kadish A: 2Kadish B: 4Kadish C: 16Kadish D: 0 | N0: 22 | 22(EEA: 7Open: 15) | NR | RT: 22 | R0: 10R+: 8R close: 4 | 36.4 | 37(5-95) | DFS (m) | HR R+ 6.17 (CI 1.05–36.08), p = 0.0436* | |
| Ungar 2024 67 | RCS(Israel 2002-2022) | 18 | 55(23-82) | 7/11 | ACC:18 | MS:8NC: 3ES: 3 | T4a: 3T4b: 15 | N0: 15N+: 3 | 18(EEA: 4Open: 14) | RT: 2 | RT: 16 | R0: 7R+: 11(R1: 6; R2: 25) | 61.1 | 240(NR) | OS (u) | p = 0.356DSS (u) | p = 0.732 | |
| Vedula 2023 68 | RCS(USA 2004-2016) | 349 | ≤ 60y: 132> 60y: 217 | 219/130 | SNAC: 349 | NC: 202ES: 88MS:59 | T1: 136T2: 77T3: 52T4: 84 | N0: 349 | 349(EEA: 93Open: 100NR: 156) | 0 | RT: 154 | R0: 222R+: 87unknown: 40 | 28.2 | NR | OS (u) | p = 0.101 | |
| Vermassen 2024 69 | RCS(Belgium 1998-2018) | 91 | #63(26-85) | 89/2 | SNAC(ITAC): 91 | NR | T1: 9T2: 37T3: 16T4a: 20T4b: 9 | N0: 91 | 91(EEA: 51Open: 40) | NR | RT: 90CHT: 1 | R0: 52R+: 39(R1: 9; R2: 30) | 42.9 | 21.5(7.7-22.1) | OS (u) | HR R+ 2.39 (CI 1.41-4.07), p = 0.0012*DSS (u) | HR R+ 2.88 (CI 1.51-5.49), p = 0.0013*DFS (u) | HR R+ 2.15 (CI 1.21-3.81), p = 0.0083*LRFS (u) | HR R+ 2.35 (CI 1.26-4.39), p = 0.0076*OS (m) | HR R+ 2.14 (CI 1.23-3.71), p = 0.0069* DSS (m) | HR R+ 2.73 (CI 1.36-5.47), p = 0.0047*DFS (m) | HR R+ 1.63 (CI 0.90-2.95) p = 0.1074LRFS (m) | HR R+ 1.94 (CI 1.01-3.73), p = 0.0475* | |
| Wang 2024 70 | RCS(China 2009-2021) | 441 | < 60y: 243≥ 60y: 190 | 310/131 | SCC: 441 | NC: 191MS: 147ES: 80other: 23 | T1-2: 44T3: 90T4: 307 | N0: 368N+: 73 | 387(EEA: 270 Open: 97) | RT-CHT: 122 | RT-CHT: 245 | R0: 127R+: 240(R1: 127; R2: 113) | 65.4 | 54(2.1-162.9) | LFR (u) | OR 2.35 (CI 1.32-4.16), p < 0.01*OS (m) | HR R2 vs R0/1 1.88 (CI 1.27-2.80), p = 0.002*PFS (m) | HR R2 vs R0/1 1.96 (CI 1.36-2.83), p < 0.01* | |
| Zafereo 2008 71 | RCS(USA 1989-2004) | 18 | 58.4(38-85) | 11/7 | ONB:18 | NR | Kadish A: 2Kadish B: 7Kadish C: 9 | N0: 14N+: 4 | 17(EEA: 3Open: 14) | RT: 2RT-CHT: 1 | RT: 6RT-CHT: 3 CHT: 1 | R0: 12R+: 2unknown: 3 | 14.3 | 66(1-312) | DSS (u) | p = 0.003* RFS (u) | p = 0.0006* | |
| Zhou 2024 72 | RCS(China 2008-2021) | 22 | 53.6(25-80) | 14/8 | SNAC(nITAC): 22 | NC: 15MS: 10ES: 7 | T1-T2: 7T3-T4: 15 | N0-N1: 20N2: 2 | 19(EEA: 9Open: 10) | CHT: 1 | RT: 6RT-CHT: 2 | R0: 15R+: 4 | 21.1 | #48.5(3-144) | OS (u) p = 0.192 | |
| ACC: adenoid cystic carcinoma; CHT: chemotherapy; Comb., combined approach; CSS: cancer specific survival; DFS: distant free survival; DMFS: distant metastasis free survival; DRFS: distant recurrence free survival; DSS: disease specific survival; EEA: endoscopic endonasal approach; ES: ethmoid sinus; F: female; FS: frontal sinus; HR: hazard ratio; ICIs: immune checkpoint inhibitors; IFN: interferon; IMPT: intesity modulated particle therapy; IQR: interquartile range; IT: immunotherapy; ITAC: intestinal type adenocarcinoma; LRC: locoregional control; LRFS: local recurrence free survival; (m), multivariate analysis; M: male; MM: mucosal melanoma; MS: maxillary sinus; MSGT: minor salivary gland tumours; NC: nasal cavity; NCDB: national cancer database; nITAC: non-intestinal type adenocarcinoma; NR: not reported; n.s.: not significant; ONB: olfactory neuroblastoma; OR: odds ratio; OS: overall survival; PFS: progression free survival; PNS: paranasal sinuses; RCS: retrospective cohort study; RFS: recurrence free survival; RR: relative risk; RRFS: regional recurrence free survival; RT: radiotherapy; RT-CHT: radio-chemotherapy; SCC: squamous cell carcinoma; SD: standard deviation; SNAC: sinonasal adenocarcinoma; SNC: sinonasal carcinoma; SNEC: sinonasal neuroendocrine carcinoma; SNUC: sinonasal undifferentiated carcinoma; SS: sphenoid sinus; TT: target therapy; (u): univariate analysis; #median; °exact number not disclosed as per NCDB use agreement. | ||||||||||||||||
| Criterion and description | No. of studies |
|---|---|
| Distinction R1/R2 | |
| Yes | 8 14,15,18,31,52,68,70,71 |
| No | 56 |
| Use of frozen sections | |
| Yes | 7 23,42,48,58,64,67,70 |
| Not reported | 57 |
| Close margin defined | |
| Yes | 8 15,20,32,44,48,54,56,67 |
| Not reported | 56 |
| Distance threshold | |
| Any | 8 |
| < 5 mm (considered close) | 5 15,32,44,48,54 |
| < 5 mm (considered R1) | 2 38,39 |
| < 2 mm (considered R1) | 1 44 |
| < 1 mm (considered R1) | 2 27,54 |
| Not reported | 56 |
| R1: microscopically infiltrated margin; R2: macroscopic residual disease. | |
| PubMed, Filters: NO | (“sinonasal” OR “nasal” OR “nose” OR “ethmoid” OR “maxillary” OR “sphenoid” OR “paranasal” OR “frontal sinus”) AND (“neoplasm” OR “cancer” OR “sinonasal neoplasm” OR “paranasal neoplasm” OR “sinonasal tract neoplasm” OR “carcinoma”[Mesh] OR “adenocarcinoma” OR “neuroendocrine carcinoma” OR “undifferentiated carcinoma” OR “olfactory neuroblastoma” OR “esthesioneuroblastoma” OR “squamous cell carcinoma” OR “neuroendocrine carcinoma” OR “melanoma” OR “adenoid cystic carcinoma” OR “salivary gland carcinoma”) AND (“margin” OR “pathological” OR “surgical margin” OR “histology” OR “margin status” OR “Margin to depth ratio”) AND (“prognosis” OR “outcome” OR “survival” OR “impact” OR “failure pattern” OR “risk-tailored”) |
| Scopus, Filters: NO | TITLE-ABS-KEY (“sinonasal” OR “nasal” OR “nose” OR “ethmoid” OR “maxillary” OR “sphenoid” OR “paranasal” OR “frontal sinus”) AND TITLE-ABS-KEY (“neoplasm” OR “cancer” OR “sinonasal neoplasm” OR “paranasal neoplasm” OR “sinonasal tract neoplasm” OR “carcinoma” OR “adenocarcinoma” OR “neuroendocrine carcinoma” OR “undifferentiated carcinoma” OR “olfactory neuroblastoma” OR “esthesioneuroblastoma” OR “squamous cell carcinoma” OR “neuroendocrine carcinoma” OR “melanoma” OR “adenoid cystic carcinoma” OR “minor salivary gland carcinoma”) AND TITLE-ABS-KEY (“margin” OR “surgical margin” OR “margin status” OR “Margin to depth ratio”) AND (“prognosis” OR “outcome” OR “survival” OR “impact” OR “failure pattern” OR “risk-tailored”) |
| Web of Science, Filters: NO | (“sinonasal” OR “nasal” OR “nose” OR “ethmoid” OR “maxillary” OR “sphenoid” OR “paranasal” OR “frontal sinus”) AND (“neoplasm” OR “cancer” OR “sinonasal neoplasm” OR “paranasal neoplasm” OR “sinonasal tract neoplasm” OR “carcinoma” OR “adenocarcinoma” OR “neuroendocrine carcinoma” OR “undifferentiated carcinoma” OR “olfactory neuroblastoma” OR “esthesioneuroblastoma” OR “squamous cell carcinoma” OR “neuroendocrine carcinoma” OR “melanoma” OR “adenoid cystic carcinoma” OR “minor salivary gland carcinoma”) AND (“margin” OR “pathological” OR “surgical margin” OR “histology” OR “margin status” OR “Margin to depth ratio”) AND (“prognosis” OR “outcome” OR “survival” OR “impact” OR “failure pattern” OR “risk-tailored”) |
References
- Ferrari M, Orlandi E, Bossi P. Sinonasal cancers treatments: state of the art. Curr Opin Oncol. 2021;33:196-205. doi:https://doi.org/10.1097/CCO.0000000000000726
- Castelnuovo P, Turri-Zanoni M, Battaglia P. Sinonasal malignancies of anterior skull base: histology-driven treatment strategies. Otolaryngol Clin North Am. 2016;49:183-200. doi:https://doi.org/10.1016/j.otc.2015.09.012
- Wang E, Zanation A, Gardner P. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol. 2019;9:S145-S365. doi:https://doi.org/10.1002/alr.22326
- Kuan E, Wang E, Adappa N. International consensus statement on allergy and rhinology: sinonasal tumors. Int Forum Allergy Rhinol. 2024;14:149-608. doi:https://doi.org/10.1002/alr.23262
- Alokby G, Casiano R. Endoscopic resection of sinonasal and ventral skull base malignancies. Otolaryngol Clin North Am. 2017;50:273-285. doi:https://doi.org/10.1016/j.otc.2016.12.005
- Schur S, Hanna E, Su S. Long-term oncological outcomes for endoscopic endonasal versus open surgical approaches for anatomically matched, locally advanced stage T4 sinonasal malignancies with skull base involvement. J Neurosurg. 2024;140:688-695. doi:https://doi.org/10.3171/2023.7.JNS23786
- Robbins K, Bradford C, Rodrigo J. Removing the taboo on the surgical violation (cut-through) of cancer. JAMA Otolaryngol Head Neck Surg. 2016;142:1010-1013. doi:https://doi.org/10.1001/jamaoto.2016.1826
- Page M, McKenzie J, Bossuyt P. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. doi:https://doi.org/10.1136/bmj.n71
- Agarwal A, Philips R, Chitguppi C. Effect of p16 status on survival outcomes in sinonasal squamous cell carcinoma. Ann Otol Rhinol Laryngol. 2023;132:917-925. doi:https://doi.org/10.1177/00034894221121401
- Al-Qurayshi Z, Liu A, Walsh J. Presentation and outcomes of non-squamous cell carcinoma sinonasal malignancies: a national perspective. Ann Otol Rhinol Laryngol. 2022;131:420-426. doi:https://doi.org/10.1177/00034894211024783
- Amit M, Tam S, Abdelmeguid A. Mutation status among patients with sinonasal mucosal melanoma and its impact on survival. Br J Cancer. 2017;116:1564-1571. doi:https://doi.org/10.1038/bjc.2017.125
- Auger S, Patel T, Ganti A. Effect of margin status and pathological grade in treatment of sinonasal mucoepidermoid carcinoma. Laryngoscope. 2020;130:E750-E757. doi:https://doi.org/10.1002/lary.28499
- Bahig H, Ehab H, Garden A. Long-term outcomes of modern multidisciplinary management of sinonasal cancers: the M.D. Anderson experience. Head Neck. 2023;45:1692-1703. doi:https://doi.org/10.1002/hed.27381
- Bristol I, Ahamad A, Garden A. Postoperative radiotherapy for maxillary sinus cancer: long-term outcomes and toxicities of treatment. Int J Radiat Oncol Biol Phys. 2007;68:719-730. doi:https://doi.org/10.1016/j.ijrobp.2007.01.032
- Chao K, Kaplan C, Simpson J. Esthesioneuroblastoma: the impact of treatment modality. Head Neck. 2001;23:749-757. doi:https://doi.org/10.1002/hed.1107
- Choby G, Geltzeiler M, Almeida J. Multicenter survival analysis and application of an olfactory neuroblastoma staging modification incorporating Hyams grade. JAMA Otolaryngol Head Neck Surg. 2023;149:837-844. doi:https://doi.org/10.1001/jamaoto.2023.1939
- Cracchiolo J, Patel K, Migliacci J. Factors associated with a primary surgical approach for sinonasal squamous cell carcinoma. J Surg Oncol. 2018;117:756-764. doi:https://doi.org/10.1002/jso.24923
- Crawford K, Jafari A, Qualliotine J. Elective neck dissection for T3/T4 cN0 sinonasal squamous cell carcinoma. Head Neck. 2020;42:3655-3662. doi:https://doi.org/10.1002/hed.26418
- Duru Birgi S, Özkaya Akagündüz Ö, Dagdelen M. Radiotherapy results in locally advanced sinonasal cancer: Turkish Society for Radiation Oncology, Head and Neck Study Group 01-005. Am J Clin Oncol. 2024;47:279-288. doi:https://doi.org/10.1097/COC.0000000000001089
- Eide J, Kshirsagar R, Brant J. A National Cancer Database analysis of sinonasal malignant myoepithelial carcinoma outcomes. Am J Rhinol Allergy. 2023;37:7-12. doi:https://doi.org/10.1177/19458924221121419
- Elsamna S, Ahsanuddin S, Mir G. Surgical margin status and survival following resection of sinonasal mucosal melanoma. Laryngoscope. 2021;131:2429-2435. doi:https://doi.org/10.1002/lary.29574
- Ferrari M, Mattavelli D, Tomasoni M. The MUSES*: a prognostic study on 1360 patients with sinonasal cancer undergoing endoscopic surgery-based treatment: *MUlti-institutional collaborative Study on Endoscopically treated Sinonasal cancers. Eur J Cancer. 2022;171:161-182. doi:https://doi.org/10.1016/j.ejca.2022.05.010
- Ganti A, Raman A, Shay A. Treatment modalities in sinonasal mucosal melanoma: a national cancer database analysis. Laryngoscope. 2020;130:275-282. doi:https://doi.org/10.1002/lary.27995
- Guazzo E, Bowman J, Porceddu S. Advanced adenoid cystic carcinoma of the skull base – The role of surgery. Oral Oncol. 2019;99. doi:https://doi.org/10.1016/j.oraloncology.2019.104466
- Guo R, Jenkins S, Johnson B. Sinonasal mucosal melanoma: role of tumor proliferative indices and pathological factors in survival. Laryngoscope. 2022;132:2350-2358. doi:https://doi.org/10.1002/lary.30240
- Harvey R, Nalavenkata S, Sacks R. Survival outcomes for stage-matched endoscopic and open resection of olfactory neuroblastoma. Head Neck. 2017;39:2425-2432. doi:https://doi.org/10.1002/hed.24912
- Herr M, Sethi R, Meier J. Esthesioneuroblastoma: an update on the Massachusetts Eye and Ear Infirmary and Massachusetts General Hospital experience with craniofacial resection, proton beam radiation, and chemotherapy. J Neurol Surg B Skull Base. 2014;75:58-64. doi:https://doi.org/10.1055/s-0033-1356493
- Hirakawa H, Hanai N, Ozawa T. Prognostic impact of pathological response to neoadjuvant chemotherapy followed by definitive surgery in sinonasal squamous cell carcinoma. Head Neck. 2016;38:E1305-1311. doi:https://doi.org/10.1002/hed.24217
- Issa K, Teitelbaum J, Smith B. Nasal cavity squamous cell carcinoma: factors associated with treatment outcomes and potential organ preservation. Am J Rhinol Allergy. 2023;37:35-42. doi:https://doi.org/10.1177/19458924221130133
- Jafari A, Shen S, Qualliotine J. Impact of margin status on survival after surgery for sinonasal squamous cell carcinoma. Int Forum Allergy Rhinol. 2019;9:1205-1211. doi:https://doi.org/10.1002/alr.22415
- Khan M, Konuthula N, Parasher A. Treatment modalities in sinonasal undifferentiated carcinoma: an analysis from the national cancer database. Int Forum Allergy Rhinol. 2017;7:205-210. doi:https://doi.org/10.1002/alr21861
- Kılıç S, Kılıç S, Baredes S. Comparison of endoscopic and open resection of sinonasal squamous cell carcinoma: a propensity score-matched analysis of 652 patients. Int Forum Allergy Rhinol. 2018;8:421-434. doi:https://doi.org/10.1002/alr.22040
- Kim G, Park H, Keum K. Adenoid cystic carcinoma of the maxillary antrum. Am J Otolaryngol. 1999;20:77-84. doi:https://doi.org/10.1016/s0196-0709(99)90015-7
- König M, Osnes T, Jebsen P. Olfactory neuroblastoma: a single-center experience. Neurosurg Rev. 2018;41:323-331. doi:https://doi.org/10.1007/s10143-017-0859-3
- König M, Osnes T, Bratland Å. Squamous cell carcinoma of the paranasal sinuses: a single center experience. J Neurol Surg B Skull Base. 2020;81:664-672. doi:https://doi.org/10.1055/s-0039-1694967
- König M, Osnes T, Bratland Å. Treatment of sinonasal adenocarcinoma: a population-based prospective cohort study. J Neurol Surg B Skull Base. 2020;81:627-637. doi:https://doi.org/10.1055/s-0039-1694050
- Konuthula N, Khan M, Parasher A. The presentation and outcomes of mucosal melanoma in 695 patients. Int Forum Allergy Rhinol. 2017;7:99-105. doi:https://doi.org/10.1002/alr.21831
- Konuthula N, Iloreta A, Miles B. Prognostic significance of Kadish staging in esthesioneuroblastoma: an analysis of the National Cancer Database. Head Neck. 2017;39:1962-1968. doi:https://doi.org/10.1002/hed.24770
- Lehrich B, Yasaka T, Goshtasbi K. Outcomes of primary versus salvage surgery for sinonasal malignancies: a population-based analysis. Laryngoscope. 2021;131:E710-E718. doi:https://doi.org/10.1002/lary.28925
- Li W, Lu H, Zhang H. Squamous cell carcinoma associated with inverted papilloma: recurrence and prognostic factors. Oncol Lett. 2020;19:1082-1088. doi:https://doi.org/10.3892/ol.2019.11185
- Li Y, Wang C, Wang R. Prognostic factors of sinonasal squamous cell carcinomas arising de novo and from inverted papilloma. Am J Rhinol Allergy. 2021;35:114-121. doi:https://doi.org/10.1177/1945892420939422
- Li J, Li B, Xu J. A retrospective review of non-intestinal-type adenocarcinoma of nasal cavity and paranasal sinus. Oncol Lett. 2023;25. doi:https://doi.org/10.3892/ol.2023.13718
- Lian M, Han B, Chen J. Investigating the impact of clinical and genetic factors on the post-surgery prognosis of sinonasal squamous cell carcinoma. Sci Rep. 2024;14. doi:https://doi.org/10.1038/s41598-024-73157-6
- Malouf G, Casiraghi O, Deutsch E. Low- and high-grade esthesioneuroblastomas display a distinct natural history and outcome. Eur J Cancer. 2013;49:1324-1334. doi:https://doi.org/10.1016/j.ejca.2012.12.008
- McMillan R, Van Gompel J, Link M. Long-term oncologic outcomes in esthesioneuroblastoma: an institutional experience of 143 patients. Int Forum Allergy Rhinol. 2022;12:1457-1467. doi:https://doi.org/10.1002/alr.23007
- Nakagawa T, Kodama S, Kobayashi M. Endoscopic endonasal management of esthesioneuroblastoma: a retrospective multicenter study. Auris Nasus Larynx. 2018;45:281-285. doi:https://doi.org/10.1016/j.anl.2017.05.001
- Nakamaru Y, Suzuki M, Kano S. The role of endoscopic resection for selected patients with sinonasal squamous cell carcinoma. Auris Nasus Larynx. 2021;48:131-137. doi:https://doi.org/10.1016/j.anl.2020.06.014
- Nakaya M, Mochiki M, Takeuchi S. Malignant melanoma of nasal cavity: report of 16 Japanese patients. Auris Nasus Larynx. 2004;31:233-237. doi:https://doi.org/10.1016/j.anl.2004.03.001
- Nalavenkata S, Sacks R, Adappa N. Olfactory neuroblastoma: fate of the neck – A long-term multicenter retrospective study. Otolaryngol Head Neck Surg. 2016;154:383-389. doi:https://doi.org/10.1177/0194599815620173
- Nishio N, Fujimoto Y, Fujii M. Craniofacial resection for T4 maxillary sinus carcinoma: managing cases with involvement of the skull base. Otolaryngol Head Neck Surg. 2015;153:231-238. doi:https://doi.org/10.1177/0194599815586770
- Ogawa K, Toita T, Kakinohana Y. Postoperative radiotherapy for squamous cell carcinoma of the maxillary sinus: analysis of local control and late complications. Oncol Rep. 2001;8:315-319. doi:https://doi.org/10.3892/or.8.2.315
- Ono T, Sakata K, Tanaka N. Salvage surgery for a locally persistent or recurrent tumor in maxillary cancer patients who have undergone radiotherapy and concomitant intra-arterial cisplatin: implications for surgical margin assessment. Int J Oral Maxillofac Surg. 2019;48:567-575. doi:https://doi.org/10.1016/j.ijom.2018.10.019
- Paré A, Blanchard P, Rosellini S. Outcomes of multimodal management for sinonasal squamous cell carcinoma. J Craniomaxillofac Surg. 2017;45:1124-1132. doi:https://doi.org/10.1016/j.jcms.2017.05.006
- Parikh A, Fuller J, Lehmann A. Prognostic impact of adverse pathologic features in sinonasal squamous cell carcinoma. J Neurol Surg B Skull Base. 2021;82:E114-E119. doi:https://doi.org/10.1055/s-0040-1710516
- Patel S, Singh B, Stambuk H. Craniofacial surgery for esthesioneuroblastoma: report of an international collaborative study. J Neurol Surg B Skull Base. 2012;73:208-220. doi:https://doi.org/10.1055/s-0032-1311754
- Patel A, Haleem A, Revercomb L. Surgical resection and overall survival in cT4b sinonasal non-squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2024;9. doi:https://doi.org/10.1002/lio2.70025
- Petruzzelli G, Howell J, Pederson A. Multidisciplinary treatment of olfactory neuroblastoma: patterns of failure and management of recurrence. Am J Otolaryngol. 2015;36:547-553. doi:https://doi.org/10.1016/j.amjoto.2015.02.008
- Qatanani A, Eide J, Harris J. The impact of delay in treatment on survival in surgically managed sinonasal undifferentiated carcinoma. J Neurol Surg B Skull Base. 2022;84:320-328. doi:https://doi.org/10.1055/s-0042-1755601
- Resto V, Eisele D, Forastiere A. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck. 2000;22:550-558. doi:https://doi.org/10.1002/1097-0347(200009)22_6<550::AID-HED2>3.0.CO;2-0
- Rojas-Lechuga M, Gras-Cabrerizo J, Avilés-Jurado F. Sinonasal mucosal melanomas: defining profiles for better survival outcomes. Rhinology. 2022;60:347-356. doi:https://doi.org/10.4193/Rhin21.251
- Sayed Z, Migliacci J, Cracchiolo J. Association of surgical approach and margin status with oncologic outcomes following gross total resection for sinonasal melanoma. JAMA Otolaryngol Head Neck Surg. 2017;143:1220-1227. doi:https://doi.org/10.1001/jamaoto.2017.2011
- Sun M, Wang K, Qu Y. Long-term analysis of multimodality treatment outcomes and prognosis of esthesioneuroblastomas: a single center results of 138 patients. Radiat Oncol. 2020;15. doi:https://doi.org/10.1186/s13014-020-01667-4
- Tajudeen B, Vorasubin N, Sanaiha Y. Sinonasal mucosal melanoma: 20-year experience at a tertiary referral center. Int Forum Allergy Rhinol. 2014;4:592-597. doi:https://doi.org/10.1002/alr.21324
- Torabi S, Spock T, Cardoso B. Multi-modality treatment and survival in sinonasal minor salivary gland tumors. J Neurol Surg B Skull Base. 2020;81:198-205. doi:https://doi.org/10.1055/s-0039-1683437
- Tsutsumi K, Ahmed K, Goshtasbi K. Impact of esthesioneuroblastoma treatment delays on overall patient survival. Laryngoscope. 2023;133:764-772. doi:https://doi.org/10.1002/lary.30136
- Tsutsumi Y, Omura K, Kijima Y. The impact of multidisciplinary approaches on the outcomes of olfactory neuroblastoma treated with postoperative radiotherapy. Cancer Med. 2024;13. doi:https://doi.org/10.1002/cam4.6943
- Ungar O, Vass R, Shapira U. Advanced stage adenoid cystic carcinoma of the sinonasal cavity and skull base: a retrospective 20-year analysis. Int J of Oral Maxillofac Surg. 2025;54:199-207. doi:https://doi.org/10.1016/j.ijom.2024.08.040
- Vedula S, Kheir L, Hu P. Adjuvant radiation and survival following surgical resection of sinonasal adenocarcinoma. Laryngoscope. 2023;133:2603-2612. doi:https://doi.org/10.1002/lary.30567
- Vermassen T, De Keukeleire S, Saerens M. Choice of surgery in intestinal-type adenocarcinoma of the sinonasal tract: a long-term comparative study. Eur Arch Otorhinolaryngol. 2024;281:2993-3004. doi:https://doi.org/10.1007/s00405-024-08447-w
- Wang L, Wang J, Wang T. Patterns of treatment failure in patients with sinonasal squamous cell carcinoma after chemoradiotherapy. Br J Radiol. 2024;97:1870-1878. doi:https://doi.org/10.1093/bjr/tqae175
- Zafereo M, Fakhri S, Prayson R. Esthesioneuroblastoma: 25-year experience at a single institution. Otolaryngol Head Neck Surg. 2008;138:452-458. doi:https://doi.org/10.1016/j.otohns.2007.12.038
- Zhou J, Zhao X, Feng L. Non-intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses: an analysis of clinical characteristics and prognosis. Eur Arch Otorhinolaryngol. 2024;281:4973-4982. doi:https://doi.org/10.1007/s00405-024-08715-9
- Baddour H, Magliocca K, Chen A. The importance of margins in head and neck cancer. J Surg Oncol. 2016;113:248-255. doi:https://doi.org/10.1002/jso.24134
- Chatelet F, Simon F, Bedarida V. Surgical management of sinonasal cancers: a comprehensive review. Cancers. 2021;13. doi:https://doi.org/10.3390/cancers13163995
- Trope M, Triantafillou V, Kohanski M. Adenoid cystic carcinoma of the sinonasal tract: a review of the national cancer database. Int Forum Allergy Rhinol. 2019;9:427-434. doi:https://doi.org/10.1002/alr.22255
- Ferrari M, Taboni S, Carobbio A. Sinonasal squamous cell carcinoma, a narrative reappraisal of the current evidence. Cancers. 2021;13. doi:https://doi.org/10.3390/cancers13112835
- Arosio A, Bernasconi D, Valsecchi M. Patterns of recurrences in sinonasal cancers undergoing an endoscopic surgery-based treatment: results of the MUSES* on 940 patients: *MUlti-institutional collaborative Study on Endoscopically treated Sinonasal cancers. Oral Oncol. 2022;134. doi:https://doi.org/10.1016/j.oraloncology.2022.106123
- Torabi S, Spock T, Cardoso B. Margins in sinonasal squamous cell carcinoma: predictors, outcomes, and the endoscopic approach. Laryngoscope. 2020;130:E388-E396. doi:https://doi.org/10.1002/lary.28315
- Patel S, Singh B, Polluri A. Craniofacial surgery for malignant skull base tumors – Report of an international collaborative study. Cancer. 2003;98:1179-1187. doi:https://doi.org/10.1002/cncr.11630
- Abdelmeguid A, Raza S, Su S. Endoscopic resection of sinonasal malignancies. Head Neck. 2020;42:645-652. doi:https://doi.org/10.1002/hed.26047
- Shah J, Levyn H, Valero C. Skull base surgery for malignant tumors: the 2nd international collaborative study (1995-2015). Head Neck. 2024;46:2762-2775. doi:https://doi.org/10.1002/hed.27746
- Tang A, Taori S, Dang S. Immunotherapy in the management of sinonasal mucosal melanoma: a systematic review. Otolaryngol Head Neck Surg. 2024;171:368-380. doi:https://doi.org/10.1002/ohn.790
- Salem A, Chen M, Williams M. Resectable sinonasal mucosal melanoma in the immunotherapy era: upfront surgery vs neoadjuvant therapy. Head Neck. Published online 2025. doi:https://doi.org/10.1002/hed.28098
- Takayesu J, Parvathaneni U, Laramore G. Adjuvant proton beam radiation therapy for sinonasal mucosal melanoma. Cancer Rep. 2025;8. doi:https://doi.org/10.1002/cnr2.70111
- Miller E, Blakaj D, Swanson B. Sinonasal adenoid cystic carcinoma: treatment outcomes and association with human papillomavirus. Head Neck. 2017;39:1405-1411. doi:https://doi.org/10.1002/hed.24778
- Lupinetti A, Roberts D, Williams M. Sinonasal adenoid cystic carcinoma: the M.D. Anderson Cancer Center experience. Cancer. 2007;110:2726-2731. doi:https://doi.org/10.1002/cncr.23096
- Amit M, Binenbaum Y, Sharma K. Analysis of failure in patients with adenoid cystic carcinoma of the head and neck. An international collaborative study. Head Neck. 2014;36:998-1004. doi:https://doi.org/10.1002/hed.23405
- Gil Z, Carlson D, Gupta A. Patterns and incidence of neural invasion in patients with cancers of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 2009;135:173-179. doi:https://doi.org/10.1001/archoto.2008.525
- Michel G, Joubert M, Delemazure A. Adenoid cystic carcinoma of the paranasal sinuses: retrospective series and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130:257-262. doi:https://doi.org/10.1016/j.anorl.2012.09.010
- Amit M, Binenbaum Y, Sharma K. Adenoid cystic carcinoma of the nasal cavity and paranasal sinuses: a meta-analysis. J Neurol Surg B Skull Base. 2013;74:118-125. doi:https://doi.org/10.1055/s-0033-1347358
- Thompson L, Penner C, Ho N. Sinonasal tract and nasopharyngeal adenoid cystic carcinoma: a clinicopathologic and immunophenotypic study of 86 cases. Head Neck Pathol. 2014;8:88-109. doi:https://doi.org/10.1007/s12105-013-0487-3
- Wiseman S, Popat S, Rigual N. Adenoid cystic carcinoma of the paranasal sinuses or nasal cavity: a 40-year review of 35 cases. Ear Nose Throat J. 2002;81:510-514.
- Amit M, Abdelmeguid A, Watcherporn T. Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. JCO. 2019;37:504-512. doi:https://doi.org/10.1200/JCO.18.00353
- Nicolai P, Schreiber A, Bolzoni Villaret A. Intestinal type adenocarcinoma of the ethmoid: outcomes of a treatment regimen based on endoscopic surgery with or without radiotherapy. Head Neck. 2016;38:E996-E1003. doi:https://doi.org/10.1002/hed.24144
- Bastier P, De Gabory L. Design and assessment of an anatomical diagram for sinonasal malignant tumor resection. Rhinology. 2016;54:361-367. doi:https://doi.org/10.4193/Rhino15.355
- Ferrari M, Gaudioso P, Taboni S. Intraoperative surgical navigation improves margin status in advanced malignancies of the anterior craniofacial area: a prospective observational study with systematic review of the literature and meta-analysis. Eur J Surg Oncol. 2025;51. doi:https://doi.org/10.1016/j.ejso.2024.109514
- Hart Z, Nishio N, Krishnan G. Endoscopic fluorescence-guided surgery for sinonasal cancer using an antibody-dye conjugate. Laryngoscope. 2020;130:2811-2817. doi:https://doi.org/10.1002/lary.28483
- Chiu A, Ma Y. Accuracy of intraoperative frozen margins for sinonasal malignancies and its implications for endoscopic resection of sinonasal melanomas. Int Forum Allergy Rhinol. 2013;3:157-160. doi:https://doi.org/10.1002/alr.21075
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1493 times
- PDF downloaded - 583 times



