Head and neck
Vol. 45: 111TH CONGRESS SIOECHCF - OFFICIAL REPORT 2025
The impact of histology and molecular biology in refining the prognostic value of surgical margins in head and neck oncology – A narrative review
Abstract
The intraoperative evaluation of surgical margins (SM) using frozen section analysis is a widely adopted practice in surgery for head and neck cancer (HNC), offering real-time pathological assessment. However, its reliability is not without challenges, as inconsistencies in specimen processing, tissue shrinkage, and interobserver variability can impact its accuracy. This review explores key aspects of SM assessment in HNC, discussing different approaches, particularly dealing with intraoperative assessment and comparing sample-driven and tumour bed-driven approaches. It examines the accuracy of these techniques and their prognostic implications, especially in cases where a positive frozen section analysis leads to revision of margins. Another important factor discussed is the impact of tissue artefacts on margin interpretation, alongside practical recommendations for assessing bone margins. A crucial point emphasised throughout is the need for strong collaboration between surgeons and pathologists to enhance the reliability of intraoperative margin evaluation.
Beyond traditional histopathological methods, recent advancements in imaging techniques are also considered. Technologies such as narrow band imaging, Raman spectroscopy, optical coherence tomography, hyperspectral imaging, computer-assisted surgery, and confocal microscopy are gaining attention for their potential to refine margin assessment and improve surgical precision. Looking ahead, the focus should be on refining intraoperative SM assessment techniques, integrating artificial intelligence-driven imaging solutions, and developing standardised guidelines through large-scale meta-analyses. A multidisciplinary approach that brings together pathology, radiology, and surgical expertise will be crucial in optimising tumour resection strategies and help to reduce the risk of local recurrence.
Introduction
Achieving an initially negative surgical margin predicts the optimal prognostic outcome for patients with head and neck squamous cell carcinoma (HNSCC), and surgical margins (SM) represent the only factor potentially controllable by the surgeon. Negative margins indicate that the tumour has been completely removed and serves as a guide for postoperative adjuvant treatments. However, the issue of margin adequacy, and the definition of “negative” margins, are objects of longstanding scientific debate. A previous American Head and Neck Society (AHNS) survey highlighted the spectrum of surgeons’ opinions regarding margin adequacy, with nearly half defining a clear margin as > 5 mm 1. In contrast, SM free from tumour but measuring < 5 mm are defined as “close”.
Patient prognostic stratification according to conventional margin status is supported by robust and large literature data, as summarised by a recent meta-analysis on 8,435 patients from 26 studies, most of whom with oral squamous cell carcinomas (OSCC) (Cover figure and Figure 1). Hamman et al. reported a significant stepwise improvement in local recurrence (LR) and overall survival (OS) relative risks as margin status categorically improved from positive to close to clear2. Stratification by margin status defined as negative, close, and positive according to the cutoff of 5 mm has been introduced in the AJCC Staging Manual (8th edition) and in international guidelines. It is important to note that, while HNSCC included in the validation studies covered all head and neck subsites 3, albeit with a disproportionate prevalence of OSCC, the AJCC formally adopted the 5 mm cutoff for close tumours only for OSCC, and that most published literature exploring its strengths and limitations focuses on this tumour site 2.
Recent studies have challenged the 5 mm cut-off to reach a more precise definition of safe margin distance and negativity threshold. A recent review summarised the results of the most relevant studies exploring optimal cutoff values for microscopic surgical distance in OSCC with reference to different outcome measures (2-, 3-, or 5-year local control [LC], OS, LR, loco-regional recurrence [LRR], loco-regional control [LRC], disease-specific survival [DSS], or loco-regional recurrence free survival [LRRFS]) 4. Significant cutoff values ranged between 1 and 7.6 mm, confirming that consensus on the extent of safe margins is far from reached. A meta-analysis in 2023 including 7 studies concluded that margins between 4 and 4.9 mm had a similar LR risk ratio compared with margins ≥ 5 mm, while margins < 4 mm had significantly higher risks 5. No pooled data are available for other outcome parameters, including survival, due to the high heterogeneity of the available studies. Interestingly, recent studies have found that treating SM as continuous, rather than categorical, variables improves the correlation with survival 6,7, as well as with recurrence 8.
Controversies exist on how to treat positive (and possibly close) margins, be it by re-excision in case of intraoperative identification of positive margins, or adjuvant therapy, as originally proposed by the EORTC and RTOG studies 9,10. This is mostly due to contradictory results obtained by works aimed at assessing the prognostic impact of such decisions. There are several technical reasons beyond intrinsic tumour behaviour that may explain the lack of robust evidence in this field; among them, the limited standardisation of sample handling by surgeons and pathologists and of tissue processing plays a crucial role: in particular, it is virtually impossible to quantify and standardise the amount of thermal damage and shrinkage suffered by the tissue in the pre-analytical phases. Moreover, the approach to the evaluation of SM is influenced by the practice of the surgical team and pathologists, with particular reference to the sampling of margins from the tumour bed or from the resection specimen and could benefit from the application of recently developed technologies, including artificial intelligence (AI).
We will cover the above topics in the following sections to provide an overview of the status of margin assessment in HNSCC.
Dysplastic margins
The impact of dysplasia at margins of HNSCC resection is not well understood. Most respondents to the AHNS survey 1 considered carcinoma in situ as a positive margin, and 76% considered dysplasia at the margin as a negative SM. On the other hand, the College of American Pathologists guidelines recommend that moderate and severe dysplasia at SM should be considered as positive margins 11. A recent large retrospective study on 565 resected OSCCs showed that dysplastic and close SM did not significantly affect LRR or OS in comparison with negative SM 12; however, patients with severe dysplasia at margins had a higher recurrence rate than those with mild and moderate dysplasia, even if more than half received adjuvant radiation therapy 12. Other studies reported that dysplastic SM was not a significant risk factor in OSCC and that the severity of dysplasia had no impact 13. Senarath et al. 14 recently reviewed articles correlating outcomes with the presence of dysplastic margins and concluded that moderate to severe dysplasia at the margin required re-excision. Overall, due to the lack of definite evidence, surgeons tend to address SM with high-grade dysplasia on a case-by-case basis, highlighting the need for definite guidelines on this topic and the importance that pathologists precisely document the presence and grade of dysplasia on margins.
Margins at extra-oral sites
There is a lack of conclusive evidence regarding SM beyond the oral cavity. The anatomical differences among subsites in the head and neck region make it difficult to directly compare the clinical significance of a margin greater than 5 mm across these areas 15. A key question remains: is achieving a margin greater than 5 mm feasible in the oropharynx, larynx, and hypopharynx? Several studies have indicated that the anatomical constraints of the head and neck region limit SM due to the thinness of various tissue layers 16. In the literature, only a limited number of studies have specifically addressed SM in total laryngectomy/laryngopharyngectomy procedures 17-23 (Fig. 2). Among them, only one adhered to the Royal College of Pathologists (RCP) guidelines 24. Two studies classified close margins as being less than 5 mm and considered margins as positive when tumour cells were present at the resection surface 22-25. The remaining studies described margin status using qualitative terms rather than precise numerical values, employing classifications such as ‘positive’, ‘microscopically positive’, ‘tumour at the resection surface’, ‘negative’, ‘safe margins’, or ‘no invasive tumour at the resection surface’. Due to these inconsistencies, determining the clinical relevance of SM in the larynx and hypopharynx remains challenging. Achieving consensus on the prognostic significance of SM at these sites would require meta-analyses of studies that share standardised definitions, methodologies, and postoperative treatment strategies 26. From a practical point of view, for glottic cancers a smaller margin of 1-2 mm is considered sufficient with particular regards to transoral laser resections 27,28. As part of the Department of Veterans Affairs Laryngeal Cancer Study, one paper reported 50% survival outcomes for clear margins, 57% for close (less than 5 mm) margins, and 27% for involved margins in laryngeal SCC 29. In transoral endoscopic and robotic approaches for oropharyngeal cancers, margins of 1.5-2 mm are considered acceptable4. In hypopharyngeal tumour, submucosal patterns of spread could predispose to inadequate margins 30. A study has shown that the propensity for submucosal tumour extension was least in the superior direction (3-10 mm) and highest in the medial (2-37 mm), lateral (2-37 mm), and inferior direction (3-35 mm) 31. The same group has also emphasised adequate radial clearance and suggested that a radial clearance of less than 1 mm is an independent prognostic factor for OS, DFS, and regional RFS 32. Hence, greater margins up to 30 mm have been recommended in hypopharyngeal tumours. To integrate the minimal free margin proposed by the Royal College of Pathologists guidelines and establish it as a standard of care for laryngeal and hypopharyngeal cancer, further investigation into its clinical relevance is essential.
Intraoperative evaluation of tumour margins
The intraoperative assessment of SM by frozen section analysis is a widely accepted and commonly utilised approach in HNSCC surgery. Its reliability and clinical value have been well demonstrated. The 2024 NCCN guidelines report: ‘Frozen section margin assessment is always at the discretion of the surgeon and should be considered when it will facilitate complete tumour removal. Achieving adequately wide margins may require resection of an adjacent structure in the oral cavity or laryngopharynx such as the base of the tongue and/or anterior tongue, mandible, larynx, or portions of the cervical oesophagus. This technique enables rapid pathological evaluation during surgery 33. Ideally, margin assessment should follow a uniform, standardised approach for all cases. However, specific standards for head and neck procedures have not yet been established. Findings from the survey “Critical evaluation of frozen section margins in head and neck cancer resections” indicate that there is no universally standardised approach for frozen section margin analysis in head and neck surgeries. According to the responses, 32% of participants reported using multiple processes for intraoperative margin assessment, while 18% stated that more than one method was applied for final margin evaluation, although this step was determined by the pathologist. The approach often varies by institution and tends to be internally developed to fit specific institutional practices 34. Additionally, many pathologists face uncertainty regarding the exact areas sampled for frozen section analysis and the margins that require further assessment. To address these challenges, various intraoperative techniques and strategies for SM evaluation have been developed 35 (Tab. I).
Defect-driven vs sample-driven
There is currently no evidence-based data confirming that intraoperative margin assessment improves local control. Conducting research is challenging due to the variability in margin sampling, evaluation, and reporting; however, it is clear that margins can be assessed from the specimen, referred to as specimen margins or sample-driven margins (SDM), or from the tumour bed, known as tumour bed margins (TBMs) or defect-driven margins 1,34,36. In the SDM approach, the entire resected specimen is sent to the pathologist for margin assessment. Conversely, in the defect-driven approach, the surgeon samples the tumour bed, collecting margins from the wound, or cavity, after completing the resection. In the latter method, the selection of TBMs, including their size, location, and quantity, is not directly guided by the formal margin assessment of the resected specimen. The debate extends beyond the method of margin sampling to the reliability of peripheral margin interpretation. In fact, there is a weak correlation between margins taken from the surgical bed and those directly sampled from the resected specimen. Most studies on margin assessment overlook the distinction between these two approaches and fail to consider their relationship. Research suggests that prognostically significant margins are those derived from the actual resection specimen, while tumour bed sampling alone has been associated with poorer local control 37,38. In a retrospective multicentre cohort study, Maxwell et al. found that among 95 patients relying solely on TBMs, the sensitivity was just 24.2% (95% CI: 16-34%), whereas the specificity reached 92% (95% CI: 85-97%) when SM were used as the reference standard 39. Similarly, Buchakjian et al. reported that TBMs had a 35% sensitivity in detecting positive specimen margins 40. Another study by Chang et al. found that TBMs failed to identify positive glossectomy margins in 67% of cases 37.
In evaluating the two types of margins, an economic aspect also emerged. The assessment of intraoperative margins has been associated with prolonged surgical times and increased costs 41. Moreover, these additional expenses can extend beyond the operating room, as false negative results may necessitate further therapeutic escalation 42. The current 2024 NCCN guidelines do not specify which type of margin between SDM and TBM is best to use intraoperatively with frozen sections 33.
TBM sampling remains widely used because of its efficiency and ease of execution, enabling surgeons to influence the pathology report. In a 2021 AHNS survey conducted among head and neck surgeons, 45% still favoured the tumour bed sampling method 43. This preference may stem from its technical advantages, as it is a faster approach that allows surgeons greater control over the processing of pathological specimens. However, a survey conducted among Canadian surgeons highlights that a slightly higher number of surgeons preferred a tumour-driven approach 44.
Over the years, efforts have been made to refine this technique by incorporating TBM orientation. Van Lanschot et al. explored the feasibility of a similar approach using paired markers placed on both the specimen and the surgical bed. The primary specimen underwent intraoperative analysis by both surgeon and pathologist. Based on these assessments, additional resections were carried out. As a result, margin status improved in 28 of 31 cases, and surgeons reported that identifying the positive SDM from the specimen to the wound bed became easier 45.
Villemure-Poliquin et al. evaluated the accuracy of an oriented TBM technique in comparison to an SDM technique 46. Their findings indicated that the use of an oriented TBM approach did not negatively impact LC, LRC, or DFS when compared to SDM. Moreover, the oriented TBM method demonstrated higher sensitivity than the SDM technique, with rates of 50% and 30%, respectively. The effectiveness of this approach improved when performed by the surgeon who originally developed the technique, suggesting that its application may vary depending on the operator’s experience and consistency. These findings are favourable compared to previous studies, which reported lower sensitivity values, ranging between 15% and 32% 39-47 (Tab. II).
Accuracy assessment
The interpretation of a negative TBM remains unclear 48. Unlike SDM, which can be categorised as positive, close, or negative, TBM is assessed in a binary manner, either positive or negative. Additionally, frozen sections taken from the tumour bed do not allow precise distance measurements as it would be on a resected specimen, and for this reason interpreting frozen margins remains challenging. If an SDM is negative and the corresponding TBM is also negative, the margin is unequivocally classified as negative. However, ambiguity arises in other situations. For instance, if a specimen has a close margin (ranging from 2 to 5 mm) but a negative TBM, should it still be considered a close margin? Similarly, if a TBM is initially positive but turns negative after multiple reassessments, should that margin be classified as negative or close in the final pathology report, provided that the latter scenario could result in escalated therapy? Currently, no standardised guidelines exist to categorise such cases, and adjuvant treatment decisions vary across institutions.
Prognostic impact of margin revision after positivity on frozen section analysis
A highly debated topic concerns the impact and management of SM, either SDM or TBM, that were initially positive at intraoperative frozen sections but later revised to negative. Although identifying positive margins intraoperatively and immediately resecting occult disease may seem logical, a definitive prognostic benefit and therapeutic significance of these revised margins continues to be a subject of discussion 49. Therefore, although many surgeons attempt further tissue resection when encountering a positive intraoperative margin, it remains unclear whether this additional resection significantly affects prognosis 39,48,49. Previous evidence suggests that survival rates and local tumour control among patients who undergo additional resections to achieve negative margins are comparable to those who achieve negative margins on the first attempt 50-52. Zhang et al. found that immediate further resection guided by frozen section analysis could enhance oncologic outcomes in patients with positive main specimen margins 51. Additionally, they confirmed the poor sensitivity (17%) of TBMs in predicting the status of the margins of the main specimen 51. However, the study included patients with diverse clinical characteristics, such as those who did or did not undergo adjuvant therapy and neck dissection, those at different tumour stages, and those with an uncertain SM status.
On the other hand, some researchers have suggested that re-resections provide limited benefit in reducing LRs and that tumours with an initial cut-through SM often exhibit more aggressive biological behaviour compared to those with an initially negative SM 53. Patients with initially positive margins who undergo additional resections still tend to have a worse prognosis compared to those with negative margins from the outset. A meta-analysis of retrospective studies assessing LRFS in early-stage OSCC found that revising initially positive SM to negative margins using frozen section guidance does not yield the same outcome as having clear margins from the outset and does not significantly enhance LC 53. Additionally, Bulbul et al. demonstrated that patients requiring additional resection were 2.9 times more likely to experience recurrence than those with initially negative margins, even if the final resection resulted in what was considered a negative margin 53. Buchakjian et al. compared LC in patients with positive main specimen margins who underwent additional tissue resection and found that once a positive margin was encountered on the main specimen, additional tissue resection did not impact LC. The risk of LR was lowest when SM were initially negative, higher when positive margins were revised to negative, and highest when final margins remained positive 54. Guillemaud et al. conducted a study on 65 patients, showing that the presence of tumour cut-through with positive frozen margins was associated with poorer LC, regardless of the final margin status after additional resection and revision 55. Similarly, Patel et al. found that microscopic tumour with positive frozen margins remained a negative prognostic factor even when revised to negative margins 49. Coutu et al. reported a cohort study indicating that an initial positive margin was an independent predictor of reduced survival and poorer LC, irrespective of the outcomes of additional resection 56. Recently, Kim et al. investigated the oncologic impact of revised negative SM (R1-R0) in a cohort of 441 patients with early-stage (pT1-2/N0) OSCC who underwent surgery without adjuvant therapy. In their retrospective analysis, LR was significantly higher (p = 0.045) in the R1-R0 group (13.1%) than in the R0 group (5.5%), and LRFS was better in the R0 group; however, there were no significant differences in overall recurrence or OS between these two groups. In their multivariate analysis, the authors identified an initial positive margin as an independent risk factor for LR (HR = 2.2; 95% CI, 1-4.9; p = 0.043). A revised clear SM after an initial cut-through margin increased the risk of LR in early-stage OSCC, emphasizing the need for caution in managing these patients 57.
Overall, these studies reinforce the idea that the presence of a positive margin during the initial resection negatively affects prognosis and may not be fully corrected by further resection to achieve control rates comparable to those of patients with initially negative margins. Several hypotheses have been proposed to explain why additional tissue resection may not improve survival or LC. If a positive margin is not the result of a surgical error but rather due to diffuse tumour infiltration and the absence of a clearly defined tumour edge, this could indicate an underlying tumour biology that is less responsive to primary surgical treatment 56. Moreover, studies have shown that the prognostic significance of positive margins during the initial resection is influenced by the presence of regional metastases. This further reinforces the idea that individual tumour biology plays a role in modifying the impact of a positive margin 49. Secondly, identifying the precise location of a positive margin after tumour resection can be challenging. Given the low sensitivity of conventional tumour bed sampling techniques, it is difficult to ensure that an adequate revision has been achieved or that the re-resection has been performed in the appropriate location. The spatial relationship between the resected specimen and the revised margin plays a crucial role in ensuring proper margin revision. Studies have indicated that, when a frozen section is positive, surgeons often struggle to precisely identify the corresponding site for additional sampling 39,47-49. Previous research has shown that accurately re-orienting the surgical specimen is difficult, often resulting in an error exceeding 10 mm 37,58,59. Another challenge in margin assessment is tissue shrinkage, which varies depending on the resection site (e.g., dorsal tongue or hard palate vs floor of the mouth, buccal mucosa, or soft palate) 40,60 (Tab. III).
Are tissue artefacts on margins a real issue?
In 1997, Johnson et al. investigated the potential shrinkage of tumour-free margins caused by tissue fixation. By simulating margins on the benign oral mucosa of dogs, they observed a reduction of 30-47% when comparing in vivo, post-fixation, and slide preparation measurements. However, the use of non-human subjects and non-malignant tissue raises concerns about the applicability of these findings to human OSCC and surrounding mucosa 54. Mistry et al. examined margin shrinkage in the oral cavity 30 minutes after tumour resection, reporting an average reduction of 23%, but did not account for changes following formalin fixation 61. Cheng et al. retrospectively analysed pre-excision margins in oral cavity specimens and compared them to the final histopathologic margins, finding an average reduction of 59%, and showed that this shrinkage occurs immediately post-resection and, to a lesser degree, following formalin fixation 62. Several other factors may also influence the extent of tissue shrinkage, including the resection technique used, the time elapsed before transferring the specimen to a formalin container (cold ischaemia), and delays in tissue processing 63, but also intrinsic contractility of mucosal tissues. Umstattd et al. analysed shrinkage in OSCC along the pre-analytical phase, evaluating tumour and margin measurements at different stages: in vivo, post-resection, and post-formalin fixation. Their findings indicate that the most significant shrinkage occurs between pre- and post-excision measurements, while no significant changes were observed between post-excision and post-formalin fixation. Given that the primary alterations in measurement take place before fixation, it is suggested that tissue shrinkage is predominantly due to inherent tissue properties rather than the effects of formalin processing 64. Additionally, it appears to vary across different regions within the same specimen and to affect less neoplastic than normal tissue; consequently, SM tend to move closer to the tumour mass 65. This difference is likely due to the infiltrative nature of tumour tissue. The variation in shrinkage can be explained by the distinct structural properties of the tissues. The intra-neoplastic stroma is often characterised by dense collagenisation, which creates a compact and rigid framework that resists formalin-induced shrinkage more effectively than the more flexible and loosely structured normal tissues. Studies have shown that shrinkage is more pronounced in the tongue (average shrinkage of 23.5%) compared to buccal mucosa (21.2%) 61. In a recent study, Michcik et al. introduced the term macroscopic resection margins (MRM) to eliminate clinical ambiguities in their research. MRM refers to the visibly unchanged tissue surrounding the resected OSCC, extending from the tumour’s outer edge to the surgical cut line. Their findings suggest that variations in MRM size are influenced by the specific tumour location. Notably, tongue tumours exhibited the most significant reduction in margins, with a median shrinkage of 1.5 mm. In comparison, tumours located in the floor of the mouth and maxilla showed slightly less shrinkage, with median values of 0.8 and 0.9 mm, respectively 66. Additionally, tumours at earlier stages (T1/T2) tend to exhibit greater shrinkage than those at more advanced stages (T3/T4) 61.
Following surgical excision and fixation, the specimen undergoes visual assessment during the grossing process. In HNSCC, the approach to sectioning remains a topic of debate due to the absence of standardised protocols, unlike in organs such as the colon or breast. Typically, specimens are sectioned using either the “radial or perpendicular” method, which involves cutting at a right angle to the margin (Cover figure), or the “parallel or en-face” (shave margin) technique. Notably, only the first method enables precise measurement of the microscopic distance between the (inked) margin and the tumour 67. Although the grossing step may appear straightforward and systematic, it is essential to acknowledge the possibility of errors during the initial sampling stages. Additionally, individual pathologist interpretation can introduce a degree of subjectivity in the subsequent histological assessment 68. Regardless of the grossing method used for tumour processing, each subsequent histological step, including dehydration, clearing and infiltration, microtomy, haematoxylin and eosin (H&E) staining, and slide preparation, has the potential to induce tissue alterations that may affect microscopic evaluation 69.
As a result of all of the above, pathological margins are often significantly smaller than in-situ margins, which can influence the assessment of marginal status.
This brings up an important question: Can specimen shrinkage be reliably predicted? Moreover, how can this knowledge be applied to improve margin evaluation? A noteworthy study by Burns et al. explores this issue in greater detail 70. The study identified an average shrinkage of 26% following resection and processing, leading to the suggestion that margin measurements should be adjusted accordingly. Implementing this adjustment could reduce the need for overly extensive resections, particularly in cases where tumour location limits surgical options due to functional or aesthetic concerns. Additionally, it may help prevent unnecessary re-resections or adjuvant therapy in cases with close or positive margins. It is important to emphasise that this is currently just a proposal. As suggested by Sengupta et al., variations in tissue shrinkage across different institutions and laboratories should be thoroughly assessed before determining a universally optimal margin distance. Each institute and laboratory should measure and quantify tissue shrinkage while accounting for all possible influencing factors 71. If a strong inter-institutional correlation in the data is identified, the recommended margin distance could be applied universally. However, if significant discrepancies arise, tissue shrinkage should be reassessed based on influencing factors to determine an appropriate margin distance for each institution and possibly anatomical area. Additionally, the authors recommend that pathology reports on margin assessment include the percentage of shrinkage, enabling surgeons to interpret the findings with both aspects in mind. These institutional measurements could contribute to improved prognosis for HNSCC. Therefore, future studies should focus on the correlation between tissue shrinkage and the optimal margin distance required for favourable prognostic outcomes 72. Recently Michcik et al., using modern 3D imaging techniques, developed a protocol for creating virtual images of OSCC that can be utilised in various ways, including enhancing surgeon-pathologist communication, mapping and describing tumours, and presenting cases at multidisciplinary board meetings. The creation of virtual images provides nearly unlimited possibilities for conducting and repeating measurements at countless points. Compared to traditional methodologies described in the literature, the use of virtual OSCC images represents a significant breakthrough, unlocking new opportunities that were previously unavailable 66 (Tab. IV).
Suggestions on how to evaluate the bone margin using frozen sections
While frozen sections provide intraoperative histological assessment of soft tissue margins, microscopic evaluation of bone margins is generally not available within the surgical timeframe, provided that calcified bone prevents the preparation of frozen sections. As a result, the surgical planning for bone-infiltrating carcinomas relies primarily on preoperative imaging techniques, such as MRI and CT. Despite advancements in imaging technology, accurately defining the extent of bone involvement before surgery remains a challenge. Additionally, preoperative imaging data cannot be directly applied intraoperatively to distinguish malignant from healthy tissue in real time 3. As a result, uncertainty persists regarding bone infiltration, which may lead to misjudgment of the extent of carcinoma involvement, potentially causing either over- or under-treatment. Multivariate analyses have demonstrated that only medullary invasion has clinical significance, whereas cortical invasion alone does not 73-75. According to the latest NCCN guidelines, segmental resection is advised in cases of medullary space invasion. Frozen section analysis of bone marrow can be utilised intraoperatively to assist in guiding resection decisions 27. Given the preoperative challenges in assessing bone invasion and the intraoperative limitation of frozen sections to soft tissue evaluation, Nieberler et al. implemented intraoperative cytological assessment of bone (ICAB) SM as an alternative approach 3,76-78. In their latest research, ICAB showed a sensitivity of 88% and a specificity of 78.6% in determining adequate resection 3. However, its clinical implementation revealed technical and logistical challenges, including desiccation of bone margins, insufficient or excessive trabecular material, and interference from blood cells, leading to cytological samples with poor quality. To improve diagnostic accuracy, bone margins were rinsed to remove excess blood, bone dust, and floating carcinoma cells, which do not indicate true bone infiltration. It is also crucial to avoid contamination from instruments, gloves, or residual tumour tissue during transport to prevent false positive results. The quality of ICAB relies heavily on expert cytological assessment, as prior radiotherapy may alter cells morphology, and immature haematopoietic precursor cells can be misidentified as malignant. Additionally, complex margins, infiltrative carcinoma growth, and prior radiotherapy pose challenges to both frozen sections and ICAB.
Collaboration with the surgical team
At the institutional level, several measures can enhance the accuracy of SM assessment, such as dedicated head and neck pathologists, standardised synoptic reporting, uniform laboratory processing protocols, and external quality control through routine audits. To improve specimen orientation and facilitate interpretation, the Canadian surgeons survey reported employing 3D specimen orientation techniques, using various marking methods to indicate the closest representative margin 44. Another promising area of research is the integration of 3D modelling to improve communication between surgeons and pathologists. Saturno et al. 79 and Sharif et al. 80 have demonstrated the feasibility of scanning surgically resected specimens to generate virtual 3D models, which can be annotated by pathologists for real-time intra- and postoperative consultations. While this technology remains in the experimental phase and was not directly examined in this survey, it holds potential as a valuable tool for enhancing SM assessment in the future.
The use of tissue biomarkers in the evaluation of tumour margins
The rationales supporting the search for biomarkers that could indicate either absence or presence of neoplastic transformation on SM are: (1) the observation that tumour tissue and non-neoplastic mucosa bear inherent molecular differences; (2) the theory of field cancerisation in the upper aerodigestive tract mucosa, and (3) the possibility that the number of tumour cells left beyond resection margins is too small to be detected by routine histopathology (minimal residual cancer, MRC) 81. Several findings have shown that microscopically normal mucosa surrounding SCC may host molecular abnormalities shared with tumour tissue, suggesting preneoplastic transformation 82. They also supported the hypothesis that the presence of these abnormalities on SM can negatively affect prognosis and possibly explain the limited association between conventional margin stratification and patient outcomes discussed in the above sections. Recurrence rates at the primary tumour site after R0 resection range between 12% and 30% 83,84. For these reasons, morphological margin examination may be supported using ancillary techniques. These are roughly distinguished in two main groups, although significant overlap exists: molecular and immunohistochemical analyses. Both may integrate morphological examinations and contribute to clarifying SM status. Molecular techniques provide a more comprehensive description of tissues as well as of their genomic status. However, they are expensive and time-consuming, requiring many days to be performed. Immunohistochemical analyses, instead, are less expensive and require less time, representing a valid support for routine practice (Fig. 1). More recently, protein biomarkers have also been proposed for fluorescence-guided surgical identification of negative mucosal margins 85,86.
Not surprisingly, the number of biomarkers that are expressed differentially in HNSCC and normal mucosa, and that may signal the presence of tumour cells along SM, is very high, including p53, microsatellite instability, DNA ploidy, CCDN1, p16, Chk2, laminin-5, glycosylated oncofetal fibronectin, galectin-9, hypermethylated gene promoters 87-89, and overall genomic imbalance 90. A relevant number of studies have aimed to identify if the presence of any of these biomarkers on SM could predict LR (Tab. V) 76,79,82,86,88,92-122. The list of those that have been proven to significantly predict LR in small case-control series is relatively limited. Among these, the status of p53 has been most widely studied with increasingly accurate methods over time. Other promising biomarkers include overexpression of eIF4E, loss of PERP and cornulin, overall loss of heterozygosity (LOH), p16, cyclin D1, beta-catenin, MMP9 and PTHLH RNA levels, chromosome instability, and profile of genes with promoter methylation.
It is worth noting that the bulk of studies on so-called “molecular margins” was published in the first 2 decades of this century; moreover, there are no prospective studies validating the prognostic value of biomarker expression analysis on traditionally sampled HNSCC margins, and biomarkers have not yet been applied as a support to surgical or therapeutic decisions. In reviewing the literature and discussing the multitude of molecular markers that have been evaluated for use, one factor that limits their application is the low coverage of these markers within the SM, and the relatively low specificity for tumour. For the widely studied p53, the myriad of genetic variants makes it difficult to develop simple assays that can be integrated into the clinical setting and limits the utility of mutant p53 assessment. Other examples, which include the early genetic event involving LOH at 9p21 to 22, observed in approximately 70% of HNSCC cases, dysregulation of p16, either via homozygous deletion (67%) or increased promoter methylation (21%), or microsatellite instability (50-60%), illustrate that no marker is expressed homogeneously (Tab. V). Definite evidence of practical benefits in treatment decisions stemming from molecular evaluation of SM is still lacking. Possibly the most significant concern that has so far prevented the use of molecular alterations to assess the SM in clinical practice is determining a particular biomarker’s predictive value for malignant disease.
Moreover, most of the methods reported so far are not compatible with the rapid response required for margin status by clinicians. However, Goldenberg and colleagues used quantitative methylation-specific PCR (QMSP) to determine the hypermethylation pattern of p16 and MGMT promoters in the SM. Although the number of patients in this study was small, the investigators showed that QMSP analysis could be performed within a period compatible with intraoperative assessment 122. One further limitation is related to the complexity of complete tissue sampling over a large surface of marginal tissue. The concept of margin imprinting presented by Hayashi et al. 123 is interesting. Their strategy may ease the difficulty of collecting relevant SM samples that represent the entire area of irregular surgical resection.
More recently, whole genome sequences of HNSCCs available in public databases offer the opportunity for a different approach to molecular profiling of cancer, and possibly to overcome the limitations of single biomarker analysis. One recent study 124 provided a gene signature associated with positive SM in tongue SCC, possibly predicting tumour resectability and informed surgical decisions. Furthermore, a pioneering microRNA signature in peripheral blood has recently been associated with disease recurrence in OSCC 125, paving the way for the application of liquid biopsy in the future.
Non-histopathological approaches to the evaluation of tumour margins: back to the surgical bed
Evaluating tumour margins intraoperatively is crucial to ensure complete tumour resection and minimise recurrence. In recent years, significant efforts have been directed toward developing non-invasive optical imaging techniques to support clinical diagnosis and treatment. These imaging methods provide a way to analyse the structural and compositional characteristics of tissues and cells in vivo, by detecting variations in their optical properties, resulting in highly detailed images of organs and biological structures 126. Various optical imaging modalities integrating surgery have been documented in the literature, including narrow band imaging (NBI), Raman spectroscopy (RS), optical coherence tomography (OCT), hyperspectral imaging (HSI), computer-assisted surgery (CAS), and fluorescence imaging. These techniques leverage anatomical and metabolic alterations, as well as the chemical composition of tissues 127. Due to its ability to minimise patient exposure to harmful radiation while offering valuable insights into the properties of soft tissue, optical imaging has become an essential tool in clinical practice. It is widely used to identify abnormal lesions, enabling early disease detection, providing real-time visualisation of SM, assisting in tumour removal, and tracking treatment response.
NBI is a fast, noninvasive, and safe endoscopic technique that employs two specific wavelength filters, blue and green, to improve the visualisation of mucosal structures and superficial blood vessels, thus allowing the distinction of patterns of distribution between tumour and normal mucosa. With central wavelengths of 415 nm for blue light and 540 nm for green light, NBI takes advantage of haemoglobin’s strong absorption properties, causing blood vessels to appear in darker shades, such as brown or cyan, in contrast to the surrounding mucosa 128. As a result, surgeons utilise NBI to assess characteristics of intra-epithelial papillary capillary loops for early lesion detection and differentiation between benign and malignant conditions. Key features examined include the extent of vessel dilation, curvature, variations in vessel calibre and morphology, as well as colour alterations in surrounding mucosal tissue 129. Research indicates that defining the incisional margin using NBI can lower the likelihood of positive tumour margins, enhance patient survival, and decrease recurrence rates compared to standard white-light endoscopy. In a study by Tirelli et al., NBI demonstrated a sensitivity of 100% and a specificity of 88.9% in evaluating incisional margins 130.
NBI has notable limitations when evaluating deep incisional margins, as it is unable to detect submucosal tumour extension. Consequently, NBI is most effective for assessing the margins of superficial and early-stage tumours. For locally-advanced tumours, where precise evaluation of deep margins is required, fluorescence molecular imaging offers a more accurate depiction of tumour boundaries 131.
RS is a light scattering technique based on the Raman effect and is capable of generating unique spectral fingerprints that reveal the molecular composition and biochemical characteristics of tumour tissue. By reflecting histopathological changes, RS provides valuable insights for clinical applications, including early cancer detection, tumour margin assessment, identification of metastatic lymph nodes, and identification of recurrence. The method is fast, non-invasive, requires minimal sample material, does not rely on fluorescence properties, and eliminates the need for special labelling, making it a promising tool in cancer diagnostics 132. RS has certain limitations. One challenge is the difficulty in fully distinguishing tumour from normal tissue. Due to biochemical similarities between SCC and the squamous epithelium, along with changes in nucleic acid and protein levels in inflamed connective tissue, there is a risk of misdiagnosing normal epithelium or inflammatory tissue as SCC 133,134. However, studies suggest that false positive results do not impact surgical outcomes as long as a 5-10 mm margin around the tumour is excised 134. Another limitation is the lack of a standardised diagnostic threshold to differentiate cancerous from non-cancerous tissue using spectral analysis. Yan et al. in their research introduced a novel approach combining Raman imaging technology with an AI generative model to enhance intraoperative margin assessment. In their research, Raman images were converted into H&E-stained histopathological representations using an AI generative model. This approach facilitated the transformation of RS images into visual histopathological images, eliminating the need for traditional tissue section preparation and staining processes. The AI-generated H&E images provided a clear visualisation of the pathological characteristics of the tissue and demonstrated superior performance compared to conventional clinical practice, particularly in cases of HNSCC with metastatic lymph nodes or moderate differentiation 135.
HSI is a remote optical imaging technique that captures a single image across potentially hundreds of distinct wavelengths. This method provides detailed information about tissue composition, assisting surgeons in identifying tumour margins more precisely. Fabelo et al. highlighted the importance and effectiveness of HSI in detecting brain cancer in vivo and designed a visualisation system that leverages a machine learning-based classification algorithm for near-real-time guidance 136. Additionally, research on deep learning approaches applied to the same dataset revealed superior performance compared to traditional machine learning methods. Boehm et al. conducted a proof-of-concept study to evaluate whether HSI, combined with AI through a Convolutional Neural Network (CNN) and a Graph Neural Network (GNN), can improve the differentiation between healthy and malignant tissue in ex vivo head and neck tumour specimens. The goal was to determine its potential for effective margin assessment. Their study included 32 patients diagnosed with HNSCC. Following surgical resection, their specimens underwent ex vivo HSI imaging, with annotated regions used to train the CNN and GNN models. Imaging parameters were carefully optimised to enhance efficiency, allowing for a more objective and rapid evaluation of SM. Their findings suggest that integrating HSI with neural networks provides additional benefits in distinguishing cancerous from healthy tissue. Despite the relatively small dataset (32 patients), the AI models demonstrated high accuracy 137.
CAS plays a crucial role in improving margin assessment during HNC surgery. By integrating imaging techniques such as CT, MRI, and PET, CAS enables precise preoperative visualisation and delineation of tumour margins, reducing the risk of leaving residual cancerous tissue 138,139. In OSCC with mandibular involvement, CAS has shown effectiveness in controlling SM, with outcomes comparable to those of traditional surgical techniques. Some studies suggest that CAS enhances both surgical precision and postoperative functional and aesthetic results due to its accuracy in planning and executing resections 140. However, there is no conclusive evidence that cutting guides significantly improve oncological margin control in mandibular SCC resections. Research into mixed reality systems, which integrate real-time 3D imaging with CAS, is exploring ways to further improve intraoperative margin assessment, though these technologies remain in experimental phases 141,142.
In recent years, there has been growing interest in the use of fluorescent markers to enhance tumour detection and delineation during surgery, providing real-time intraoperative guidance. These markers aim to provide a clear distinction between malignant and healthy tissues, ensuring precise excision margins. An ideal fluorescent marker should selectively bind to cancer cells, accumulate sufficiently to produce fluorescence, and effectively differentiate malignant from healthy tissue. Several fluorescent markers have been studied for their potential use in HNSCC detection, including toluidine blue, ICG, 5-ALA, and IRDye800CW-conjugated anti-EGFR antibodies. Toluidine blue has seen limited recent research 143 due to concerns over specificity. While ICG is FDA- and EMA-approved 144, its effectiveness in HNSCC remains uncertain 145, although newer ICG-based markers like ONM-100 may offer better tumour visualisation. 5-ALA, already used in glioma surgery 146, has shown promise for HNSCC in recent studies 147. Meanwhile, fluorescently labeled cetuximab and panitumumab are gaining attention, with multiple ongoing clinical trials. Panitumumab-IRDye800CW has demonstrated potential for improving surgical decision-making 148. Srinivasan et al. conducted a systematic review to identify and compare fluorescent markers used for the detection and delineation of HNSCC. Among the most promising candidates, they identified 5-ALA, cetuximab-IRDye800CW, panitumumab-IRDye800CW, and targeted ICG derivatives such as ONM-100 149. Recent studies have explored ex vivo microscopy for evaluating excision margins in HNSCC. Shavlokhova et al. 150 reported a sensitivity and specificity of 99% and 95% using ex vivo imaging of oral cavity lesions. A systematic review of 6 confocal laser endomicroscopy studies 151 showed 95% sensitivity and 93% specificity, while Zanoni et al. 152 demonstrated 90% sensitivity and 98.3% specificity for in vivo reflectance confocal microscopy (Tab. VI).
Conclusions
In HNC surgery, evaluation of margin status represents an important part of tumour staging and prognostic assessment. This may be obtained in frozen and definitive sections, sometimes with the use of ancillary techniques. It should be noted, however, that the risk of LR is affected not only by the surgical excision margin. Alongside this, T category, degree of differentiation, pN, lympho-vascular, perineural, depth, and pattern of invasion are other independent risk factors for LR.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors contribuited equally to the work.
Ethical consideration
Not applicable.
History
Received: March 15, 2025
Accepted: March 21, 2025
Figures and tables
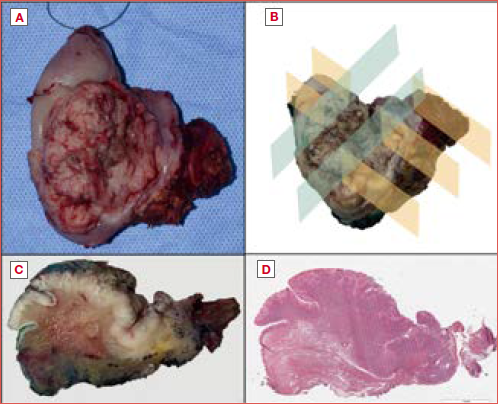
Figure 1. (A) A neoplastic lesion on the left lingual border in a male patient affected by graft-versus-host disease; (B) Tumour bed after lesion resection; (C) Whole slide image of the lesion stained with haematoxylin; (D) Immunohistochemical analysis for p53 shows a pattern of basal and suprabasal overexpression, while the resection margins are negative.
Figure 2. (A) Total laryngectomy specimen. It is crucial to assess all resection margins. Intraoperatively, frozen sections must always be performed, especially on the superior margin at the level of the vallecula/base of the tongue and the inferior margin at the level of the piriform sinuses; (B) A whole slide image (WSI) of a cross-section obtained at the tumour site in the corresponding total laryngectomy specimen; (C) Open partial horizontal laryngectomy (OPHL) Type II specimen. Proper orientation of the specimen is essential. It is important to evaluate the margins and assess thyroid cartilage infiltration, as this can influence the indication for adjuvant treatment; (D) A WSI of a cross-section obtained at the tumour site in the corresponding OPHL Type II specimen; (E) Type VI cordectomy. Close collaboration with the surgeon is essential, particularly in orienting the specimen in the operating room. In this case, the margins to be evaluated include: the posterior vocal cord margin, the inferior subglottic margin, and the superior margin towards the ventricle. The anterior and lateral margins, adjacent to the perichondrium of the thyroid cartilage, may also be positive. However, in such cases, achieving negative margins is challenging. If the posterior, inferior, or superior margins are positive, a wider endoscopic resection may be performed. However, if the anterior or lateral margins are involved and further excision is necessary, an open surgical approach is required. In general, an indication for wider endoscopic resection is given only in cases of multiple positive superficial margins or a positive deep margin. However, depending on the surgeon’s experience, an endoscopic follow-up approach may also be considered. This is because margins can appear negative intraoperatively, but due to shrinkage from formalin fixation or the effects of the carbon dioxide laser, may be positive in the final histological examination; (F) A WSI of a cross-section obtained from the cordectomy specimen.
| Key points | Description |
|---|---|
| Frozen section analysis | Widely used in head and neck cancer surgery for intraoperative margin assessment |
| NCCN guidelines | Frozen section margin assessment is at the surgeon’s discretion; wide margins may require additional tissue resection |
| Proximal and distal nerve margins | Recommended for frozen section analysis to ensure complete tumour removal |
| Reliability of frozen sections | Demonstrated to be a valuable diagnostic tool for rapid intraoperative pathological evaluation |
| Lack of standardisation | There is no universally accepted standard for frozen section margin evaluation in head and neck surgery |
| Institutional variability | Different institutions apply varying approaches, often developed internally |
| Survey findings | 32% of respondents use multiple intraoperative margin assessment methods; 18% apply more than one method for final evaluation |
| Key points | Sample-driven margins (SDM) | Defect driven or tumour-bed margins (TBM) |
|---|---|---|
| Definition | Margins assessed on the resected specimen | Margins taken from the surgical bed after resection |
| Guidance | Pathologist-driven margin assessment | Surgeon-guided margin selection |
| Correlation | Stronger correlation with true SM | Weaker correlation with actual resection specimen margins |
| Local control | Better local control | Lower local control, higher false negative rate |
| Sensitivity | Higher sensitivity in detecting positive margins | Lower sensitivity (reported between 15% and 35%) |
| Surgical time | Potentially increases operative time for thorough assessment | Faster, as it avoids waiting for detailed pathological processing |
| Economic aspect | Higher costs due to extended surgery and pathology processing | Lower initial costs, but false negatives may lead to additional treatment expenses |
| Surgeon preference | Less commonly preferred due to logistical challenges | More widely used due to efficiency and ease of execution |
| Advancement | Standardised in most pathology departments | Some methods attempt to improve accuracy by orienting TBM samples |
| Key points | Description |
|---|---|
| Negative margins | Clear margins improve prognosis in head and neck cancer |
| Surgical challenges | Obtaining negative margins in the first attempt can be difficult |
| Margin revision debate | Uncertainty exists on whether re-resection improves outcomes |
| Recurrence risk | Higher recurrence rates in patients with revised margins compared to initially negative ones |
| Tumour biology role | Patients with aggressive tumours may not benefit from additional resection |
| Re-resection challenges | Difficult to identify and accurately resect positive margins |
| Key points | Description |
|---|---|
| Specimen processing | After excision, tissue is fixed and sectioned, but standardisation in head and neck cancers is lacking |
| Tissue shrinkage | Margins shrink due to processing, with reductions up to 59%, depending on tissue type and location |
| Impact on margin assessment | Shrinkage leads to margins appearing smaller, affecting classification of negative, close, or positive margins |
| Regional variability | Tongue tissue shrinks more (23.5%) than buccal mucosa (21.2%), impacting interpretation of margins |
| Predicting shrinkage | Studies suggest measurements of margins should be adjusted to compensate for shrinkage effects |
| Institutional differences | Margin shrinkage varies across institutions; standardised reporting could improve prognostic evaluation |
| Biomarker | Method | Site | Tumour-associated defect | Cases/controls | Prevalence | Specificity (%) | Sensitivity (%) | Analysis | Significance | Notes | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP53 gene | SequencingSouthern blot | Head neck | Mutation | 5/20 | 13/25 | 5/5(100) | 12/20(60) | KM/log-rank | Yes | 82 | ||
| EIF4E protein | IICH | Head neck | Overexpression | 22/43 | 36/65 | 20/22(91) | 27/43(63) | KM/log-rank | Yes | 92 | ||
| EIF4E proteinP53 protein | IICHIICH | Larynx | OverexpressionOverexpression | 25/29 | 32/546/54 | 21/25(84)6/23(26) | 18/29(82)31/31(100) | KM/log-rankMultivariateKM/log-rankMultivariate | YesYesYesNo | 93 | ||
| TP53 geneP53 protein | Phage assayIICH | Oral cavity | MutationOverexpression | 5/6 | 6/11 | 4/5(80) | 4/6(67) | ND | 94 | |||
| Microsatellites | PCR, Gel electrophoresis | Multiple | Instability | 8/17 | 11/25 | 7/8(88) | 13/17(76) | KM/log-rankMultivariate | YesYes | 95 | ||
| P53 proteinMMP9 protein4E protein | IICHIICHIICH | Head neck | Overexpression | 14/38 | 24/5228/5227/52 | 9/14(64)11/14(78)12/14(86) | 23/38(60)21/38(55)22/38(58) | KM/Cox proportional hazard/multivariate | NoNoYes/Yes | 96 | ||
| TP53 gene | Plaque assay | Multiple | Mutation | 15/61 | 50/76 | 14/15(93) | 25/61(41) | KM/log-rank | Yes | 76 | ||
| Microsatellites | PCR, Gel electrophoresis | Multiple | Instability | 5/21 | 7/26 | 5/5(100) | 19/21(90) | KM/log-rank | Yes | 97 | ||
| DNA | DNA-image cytometry | Oral cavity | Aneuploidy | 20/20 | 16/40 | 14/20(70) | 18/20(90) | Fisher exact test | Yes | 98 | ||
| TP53 gene | PCR, Plaque assay | ND | Mutation | 13/12 | 16/25 | 11/13(85) | 7/12(58) | KM/log-rank | Yes | Deep margins also evaluated | 99 | |
| CDKN2A (p16), CCNA1, DCC genes | QMSP | Multiple | Promoter methylation | 5/22 | 11/27 | 5/5(100) | 16/22(73) | KM/log-rank | Yes | At least 1 marker positive | 100 | |
| BIRC5 proteinCD44v6 protein | IICHIICH | Larynx | OverexpressionOverexpression | 41/71 | 44/11235/112 | 26/41(63)20/41(49) | 53/71(75)56/71(79) | Cox proportional hazard/multivariate | Yes/YesYes/Yes | 101 | ||
| DNA | Interphase-FISH | Oral cavity | Polyploidy | 4/15 | 8/19 | 4/4(100) | 11/15(73) | Fisher exact test | Yes | 102 | ||
| P53 protein | IICH | Oral cavity | Overexpression | 4/15 | 8/19 | 3/4(75) | 10/15(67) | Fisher exact test | No | 103 | ||
| Cytokeratin 4 proteinCornulin protein | IICHIICH | Oral cavity and oropharynx | HypoexpressionHypoexpression | 23/23 | 23/4632/46 | 17/23(74)16/23(70) | 17/23(74)16/23(70) | KM/log-rank | YesYes | 104 | ||
| CDKN2A gene | QMSP | Tongue | Promoter methylation | 6/24 | 13/30 | 5/6(67) | 16/24(67) | KM/log-rank | Yes | 105 | ||
| TP53 gene | LigAmp | Head neck | Mutations | 15/80 | 35/95 | 9/15(60) | 54/80(67) | Log-rank | No | 106 | ||
| Chromosome 3pChromosome 9pChromosome 17pP53 protein | SequenceSequenceSequenceIICH | Oral cavity and oropharynx | LOHLOHLOHOverexpression > 5% | 16/19 | ND | NDND (62)NDND (62) | NDND (74)NDND (84) | KM/Cox proportional hazard | NoYesNoYes | 107 | ||
| TP53 geneLy-6D mRNA | LigAmpqRT-PCR | Oral cavity | MutationExpression | 43/59 | 51/102ND | NDND | NDND | KM/log-rank/multivariate | Yes/YesNo | Combined markers significant | 108 | |
| PTHLH mRNAEPCAM mRNAMMP9 mRNA | qRT-PCRqRT-PCRqRT-PCR | Multiple | Overexpression 5xOverexpression 10xOverexpression 10x | 4/51 | 5/556/5513/55 | 0/4 (0)0/4 (0)4/4(100) | 46/51(90)45/51(88)42/51(82) | KM/log-rankCox proportional hazard | NoNoYes | 79 | ||
| DCC, EDNRB, p16, KIF1A genes | QMSP | Head neck | Promoter methylation profile | 6/11 | 8/17 | 5/6(83) | 8/11(72) | N/A | NA | Tissue imprints | 109 | |
| DSPP proteinBSP proteinOPN proteinMMP-2 proteinMMP-3 proteinMMP-9 protein | IICHIICHIICHIICHIICHIICH | Oral cavity | Expression | 9/11 | 11/2013/2011/2012/2013/207/20 | 8/9(89)6/9(67)7/9(78)5/9(55)7/9(78)6/9(67) | 6/11(54)4/11(36)7/11 ((64)4/11(36)5/11(45)10/11(91) | KM/Cox proportional hazard | YesNoNoNoNoYes | 110 | ||
| P53 proteinCyclin D1 protein | IICHIICH | Multiple | OverexpressionOverexpression | 35/81 | 34/11646/116 | ND | ND | KM/Cox proportional hazard/ multivariate | NoYes/No | No association with OS | 111 | |
| DCC, EDNRB, HOXA9, KIF1A, NID2, NR2B genes | QMSP | Head neck | Promoter methylation profile | Total 65 | ND | ND | ND | Cox proportional hazard | Significant combined markers; no single | Significant on margin imprint, negative histological | 112 | |
| PAX5 gene | QMSP; ddQMSP | Head neck | Promoter methylation | 35/25 | 16/60 | ND | ND | Cox proportional hazard | Yes | Margin imprint and tissue | 86 | |
| Microsatellite | Sequence | Oral cavity | InstabilityLOH | 24/58 | 40/8276/82 | NDND | NDND | KM/log-rank | YesNo | 113 | ||
| P53 proteineIF4E Protein | IICHIICH | Oral cavity and oropharynx | OverexpressionOverexpression | 7/17 | 11/2416/24 | 4/7(57)1/7(14) | 10/17(59)15/17(88) | KM/log-rank | NoNo | 114 | ||
| Chromosome 9pChromosome 17pP53 proteinP14 proteinP15 proteinP16 protein | PCR/gel electrophoresisIICHIICHIICH | Oral cavity | LOHLOHOverexpressionOverexpressionOverexpressionOverexpression | 15/56 | 14/6517/6722/7139/7130/7130/71 | 6/13(46)6/15(40)10/15(67)11/15(73)9/15(60)15/15(100) | 44/52(85)41/52(79)44/56(78)28/56(50)35/56(62)38/56(68) | KM/Cox proportional hazard/multivariate Cox regression | Yes/YesYes/NoYes/NoNo/NoYes/NoYes/No | TP16+1 9p microsatellite significant in multivariate analysis | 115 | |
| Chromosome 1 & 7P53 | FISHIICH | Oral cavity | Copy number variationOverexpression | 11/2411/31 | 18/3512/42 | 9/11(82)4/11(36) | 15/24(62)16/31(52) | KM/log rank test/multivariate Cox regression | Yes/YesNo | 116 | ||
| βcatenin proteinβcatenin proteinβcatenin mRNA | Western blotIICHqRT-PCR | Oral cavity | OverexpressionNuclear expressionOverexpression | 26/54 | 47/80 | 21/26(81) | 28/54(52) | KM/log-rank | Yes | Pooled results | 117 | |
| Microsatellite | Sequence | Oral cavity | Instability | 26/29 | 41/55 | 22/26(85) | 10/29(34) | Logistic regression | Yes | Pre-selected MSI+ tumour | 118 | |
| P53 protein | IICH | Oral cavity | Overexpression | 16/20 | 32/72 | 14/16(87) | 38/40(95) | KM/Cox proportional hazard | Yes | Dysplastic margins | 119 | |
| PERP protein | IICH | Multiple | Loss of expression | NA | 19/46 | NA | NA | KM/log-rank | Yes | 120 | ||
| Conulin proteinKI67/MIB1 proteinISG15 protein | IICHIICHIICH | Oral cavity | IRS-score (hig/low)% positive (hig/low)IRS-score (hig/low) | 15/19 | 10/34 | 7/15(47)2/15(13)3/15(20) | 16/19(84)18/19(95)18/19(95) | Pearson Chi-square/binary logistic regression | YesNoNo | 121 | ||
| TP53 geneP53 protein | RT-PCRIICH | Oral cavity | AmplificationOverexpression | 21/19 | 38/40 | 16/21(76)19/21(90) | 14/19(74)17/19(89) | KM/Cox proportional hazard/Multivariate COX regression | NoNo | Significant association with OS | 122 | |
| TP53 geneCirculating miRNA | NGS + ddPCR | Multiple | VAFsLevel quantification | 4749 (28 both tests) | 30/4726/49 | 27/42(64)25/47(53) | 2/5(40)0 | KMKM | YesYes | Significant biomarker combination | 88 | |
| KM: Kaplan-Meier; IICH: immunohistochemistry; ND: not detailed; PCR: polymerase chain reaction; QMSP: quantitative methylation-specific PCR; FISH: fluorescent in situ hybridisation; LOH: loss of heterozygosity; qRT-PCR: quantitative retro-transcribed polymerase chain reaction; NA: not assessed; OS: overall survival; MSI: microsatellite instability; IRS: immune response score; RT-PCR: real time PCR; NGS: next generation sequencing; ddPCR: digital droplet polymerase chain reaction. | ||||||||||||
| Key points | Description |
|---|---|
| Importance of margin assessment | Intraoperative tumour margin assessment is crucial for complete tumour resection and reduction of recurrence risk |
| Optical imaging techniques | NBI, RS, OCT, HSI, CAS, and fluorescence imaging improve tumour margin visualisation |
| Narrow band imaging (NBI) | Enhances superficial tumour margin assessment but is ineffective for deep margins |
| Raman spectroscopy (RS) | Provides biochemical insights but has limitations in differentiating SCC from normal tissue |
| HyperSpectral Imaging (HSI) | Effective with AI assistance but limited by long acquisition times |
| Computer-sssisted surgery (CAS) | Improves preoperative planning but lacks conclusive oncological margin control evidence |
| Fluorescence imaging | Several markers (5-ALA, cetuximab-IRDye800CW, etc.) show promise for intraoperative tumour delineation |
| Ex vivo microscopy | Studies demonstrate high sensitivity and specificity in SCC margin assessment |
References
- Meier J, Oliver D, Varvares M. Surgical margin determination in head and neck oncology: current clinical practice. The results of an international American Head and Neck Society member survey. Head Neck. 2005;27:952-958. doi:https://doi.org/10.1002/hed.20269
- Hamman J, Howe C, Borgstrom M. Impact of close margins in head and neck mucosal squamous cell carcinoma: a systematic review. Laryngoscope. 2022;132:307-321. doi:https://doi.org/10.1002/lary.29690
- Nieberler M, Stimmer H, Rasthofer D. Defining secure surgical bone margins in head and neck squamous cell carcinomas: the diagnostic impact of intraoperative cytological assessment of bone surgical margins compared with preoperative imaging. Oral Oncol. 2020;102. doi:https://doi.org/10.1016/j.oraloncology.2020.104579
- Chen Y, Zhong N, Cao L. Surgical margins in head and neck squamous cell carcinoma: a narrative review. Int J Surg. 2024;110:3680-3700. doi:https://doi.org/10.1097/JS9.0000000000001306
- Young K, Bulosan H, Kida C. Stratification of surgical margins distances by the millimeter on LR in oral cavity cancer: a systematic review and meta-analysis. Head Neck. 2023;45:1305-1314. doi:https://doi.org/10.1002/hed.27339
- Singh A, Mishra A, Singhvi H. Optimum surgical margin in squamous cell carcinoma of the oral tongue: is the current definition adequate?. Oral Oncol. 2020;111. doi:https://doi.org/10.1016/j.oraloncology.2020.104938
- Nason R, Binahmed A, Pathak K. What is the adequate margin of surgical resection in oral cancer?. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:625-629. doi:https://doi.org/10.1016/j.tripleo.2008.11.013
- Dudkiewicz D, Yosefof E, Shpitzer T. Rethinking surgical margins: a new approach to predict outcomes in oral squamous cell carcinoma. Laryngoscope. 2025;135:161-167. doi:https://doi.org/10.1002/lary.31744
- Cooper J, Zhang Q, Pajak T. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84:1198-1205. doi:https://doi.org/10.1016/j.ijrobp.2012.05.008
- Bernier J, Cooper J, Pajak T. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27:843-850. doi:https://doi.org/10.1002/hed.20279
- Kim Y, Lee C, Heo Y. Impact of dysplasia at surgical margins on oncologic outcome after curative resection of oral tongue squamous cell carcinoma: significance of high-grade dysplastic surgical margins. Eur Arch Otorhinolaryngol. 2024;281:441-449. doi:https://doi.org/10.1007/s00405-023-08233-0
- Chen T, Chang H, Yang T. Impact of dysplastic surgical margins for patients with oral squamous cell carcinoma. Oral Oncol. 2019;97:1-6. doi:https://doi.org/10.1016/j.oraloncology.2019.07.015
- Senarath N, Jayasooriya P, Siriwardena B. Epithelial dysplasia at excision margins of oral squamous cell carcinoma: a review on relationship to clinicopathological parameters and prognosis. Asian Pac J Cancer Prev. 2021;22:2313-2321. doi:https://doi.org/10.31557/APJCP.2021.22.8.2313
- Bernard S, van Lanschot C, Hardillo J. A new proposal for adequate surgical margins in larynx and hypopharynx tumor surgery-are the rcp guidelines feasible?. Cancers. 2024;16. doi:https://doi.org/10.3390/cancers16112058
- Hinni M, Zarka M, Hoxworth J. Margin mapping in transoral surgery for head and neck cancer. Laryngoscope. 2013;123:1190-1198. doi:https://doi.org/10.1002/lary.23900
- Kutter J, Lang F, Monnier P. Transoral laser surgery for pharyngeal and pharyngolaryngeal carcinomas. Arch Otolaryngol Head Neck Surg. 2007;133:139-144. doi:https://doi.org/10.1001/archotol.133.2.139
- Bernard S, van Lanschot C, Sewnaik A. Clinical relevance of surgical margins in patients with total laryngectomy or laryngopharyngectomy. Cancers. 2024;16. doi:https://doi.org/10.3390/cancers16112038
- Bich T, Vuong N, Cam Tu N. Long-term survival of patients after total pharyngolaryngoesophagectomy with gastric pull-up reconstruction for hypopharyngeal or laryngeal cancer invading cervical esophagus. Ann Otol Rhinol Laryngol. 2023;132:511-518. doi:https://doi.org/10.1177/00034894221098802
- Mazerolle P, Philouze P, Garrel R. Oncological and functional outcomes of trans-oral robotic surgery for pyriform sinus carcinoma: a French GETTEC group study. Oral Oncol. 2018;86:165-170. doi:https://doi.org/10.1016/j.oraloncology.2018.09.014
- Omura G, Ando M, Saito Y. Disease control and clinicopathological prognostic factors of total pharyngolaryngectomy for hypopharyngeal cancer: a single-center study. Int J Clin Oncol. 2015;20:290-297. doi:https://doi.org/10.1007/s10147-014-0709-z
- Bova R, Goh R, Poulson M. Total pharyngolaryngectomy for squamous cell carcinoma of the hypopharynx: a review. Laryngoscope. 2005;115:864-869. doi:https://doi.org/10.1097/01.MLG.0000158348.38763.5D
- Grasl S, Frommlet F, Faisal M. A new nomogram to predict oncological outcome in laryngeal and hypopharyngeal carcinoma patients after laryngopharyngectomy. Eur Arch Otorhinolaryngol. 2023;280:1381-1390. doi:https://doi.org/10.1007/s00405-022-07668-1
- Wulff N, Andersen E, Kristensen C. Prognostic factors for survival after salvage total laryngectomy following radiotherapy or chemoradiation failure: a 10-year retrospective longitudinal study in eastern Denmark. Clin Otolaryngol. 2017;42:336-346. doi:https://doi.org/10.1111/coa.12726
- Spector J, Sessions D, Lenox J. Management of T3N1 glottic carcinoma: therapeutic outcomes. Laryngoscope. 2006;116:106-110. doi:https://doi.org/10.1097/01.mlg.0000184767.62682.3d
- Saraniti C, Speciale R, Gallina S. Prognostic role of surgical margins in open oncologic laryngeal surgery: survival analysis of a cohort of 139 patients affected by squamous cell carcinoma. Braz J Otorhinolaryngol. 2019;85:603-610. doi:https://doi.org/10.1016/j.bjorl.2018.04.012
- Nakayama M, Holsinger C, Okamoto M. Clinicopathological analyses of fifty supracricoid laryngectomized specimens: evidence base supporting minimal margins. ORL J Otorhinolaryngol Relat Spec. 2009;71:305-311. doi:https://doi.org/10.1159/000261836
- Fiz I, Mazzola F, Fiz F. Impact of close and positive margins in transoral laser microsurgery for Tis-T2 glottic cancer. Front Oncol. 2017;7. doi:https://doi.org/10.3389/fonc.2017.00245
- Bradford C, Wolf G, Fisher S. Prognostic importance of surgical margins in advanced laryngeal squamous carcinoma. Head Neck. 1996;18:11-16. doi:https://doi.org/10.1002/(SICI)1097-0347(199601/02)18:1<11::AID-HED2>3.0CO;2-1
- Ho C, Ng W, Lam K. Submucosal tumor extension in hypopharyngeal cancer. Arch Otolaryngol Head Neck Surg. 1997;123:959-965. doi:https://doi.org/10.1001/archotol.1997.01900090073010
- Wei W. The dilemma of treating hypopharyngeal carcinoma: more or less. Hayes Martin Lecture. Arch Otolaryngol Head Neck Surg. 2002;128:229-232. doi:https://doi.org/10.1001/archotol.128.3.229
- Ho C, Ng W, Lam K. Radial clearance in resection of hypopharyngeal cancer: an independent prognostic factor. Head Neck. 2002;24:181-190. doi:https://doi.org/10.1002/hed.10002
- NCCN Guidelines Version 4.2024 Head and Neck Cancers.
- Black C, Marotti J, Zarovnaya E. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. 2006;107:2792-2800. doi:https://doi.org/10.1002/cncr.22347
- Shapiro M, Salama A. Margin analysis: squamous cell carcinoma of the oral cavity. Oral Maxillofac Surg Clin North Am. 2017;29:259-267. doi:https://doi.org/10.1016/j.coms.2017.03.003
- Hinni M, Ferlito A, Brandwein-Gensler M. SM in head and neck cancer: a contemporary review. Head Neck. 2013;35:1362-1370. doi:https://doi.org/10.1002/hed.23110
- Chang A, Kim S, Duvvuri U. Early squamous cell carcinoma of the oral tongue: comparing margins obtained from the glossectomy specimen to margins from the tumor bed. Oral Oncol. 2013;49:1077-1082. doi:https://doi.org/10.1016/j.oraloncology.2013.07.013
- Baddour H, Magliocca K, Chen A. The importance of margins in head and neck cancer. J Surg Oncol. 2016;113:248-255. doi:https://doi.org/10.1002/jso.24134
- Maxwell J, Thompson L, Brandwein-Gensler M. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141:1104-1110. doi:https://doi.org/10.1001/jamaoto.2015.1351
- Buchakjian M, Tasche K, Robinson R. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142:1191-1198. doi:https://doi.org/10.1001/jamaoto.2016.2329
- DiNardo L, Lin J, Karageorge L. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110:1773-1776. doi:https://doi.org/10.1097/00005537-200010000-00039
- Horwich P, MacKay C, Bullock M. Specimen oriented intraoperative margin assessment in oral cavity and oropharyngeal squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2021;50. doi:https://doi.org/10.1186/s40463-021-00501-5
- Bulbul M, Zenga J, Tarabichi O. Margin practices in oral cavity cancer resections: survey of American Head and Neck Society members. Laryngoscope. 2021;131:782-787. doi:https://doi.org/10.1002/lary.28976
- Daniel R, Yan B, Chandarana S. SM definition and assessment in head and neck oncology: a cross-sectional survey of Canadian Head and Neck Surgeons. J Otolaryngol Head Neck Surg. 2024;53. doi:https://doi.org/10.1177/19160216241296121
- van Lanschot C, Mast H, Hardillo J. Relocation of inadequate surgical margins in the wound bed during oral cavity oncological surgery: a feasibility study. Head Neck. 2019;41:2159-2166. doi:https://doi.org/10.1002/hed.25690
- Villemure-Poliquin N, Roy ÈM, Nguyen S. Tumor bed margins versus specimen margins in oral cavity cancer: too close to call?. J Otolaryngol Head Neck Surg. 2024;53. doi:https://doi.org/10.1177/19160216241278653
- Prabhu A, Sturgis C, Lai C. Improving margin revision: characterization of tumor bed margins in early oral tongue cancer. Oral Oncol. 2017;75:184-188. doi:https://doi.org/10.1016/j.oraloncology.2017/10.013
- Kubik M, Sridharan S, Varvares M. Intraoperative margin assessment in head and neck cancer: a case of misuse and abuse?. Head Neck Pathol. 2020;14:291-302. doi:https://doi.org/10.1007/s12105-019-01121-2
- Patel R, Goldstein D, Guillemaud J. Impact of positive frozen section microscopic tumor cut-through revised to negative on oral carcinoma control and survival rates. Head Neck. 2010;32:1444-1451. doi:https://doi.org/10.1002/hed.21334
- Kwok P, Gleich O, Hübner G. Prognostic importance of “clear versus revised margins” in oral and pharyngeal cancer. Head Neck. 2010;32:1479-1484. doi:https://doi.org/10.1002/hed.21349
- Zhang L, Judd R, Zhao S. Immediate resection of positive margins improves local control in oral tongue cancer. Oral Oncol. 2023;141. doi:https://doi.org/10.1016/j.oraloncology.2023.106402
- Patel R, Clark J, Dirven R. Prognostic factors in the surgical treatment of patients with oral carcinoma. ANZ J Surg. 2009;79:19-22. doi:https://doi.org/10.1111/j.1445-2197.2008.04791.x
- Bulbul M, Tarabichi O, Sethi R. Does clearance of positive margins improve local control in oral cavity cancer? A meta-analysis. Otolaryngol Head Neck Surg. 2019;161:235-244. doi:https://doi.org/10.1177/0194599819839006
- Buchakjian M, Ginader T, Tasche K. Independent predictors of prognosis based on oral cavity squamous cell carcinoma surgical margins. Otolaryngol Head Neck Surg. 2018;159:675-682. doi:https://doi.org/10.1177/0194599818773070
- Guillemaud J, Patel R, Goldstein D. Prognostic impact of intraoperative microscopic cut-through on frozen section in oral cavity squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2010;39:370-377.
- Coutu B, Ryan E, Christensen D. Positive margins matter regardless of subsequent resection findings. Oral Oncol. 2022;128. doi:https://doi.org/10.1016/j.oraloncology.2022.105850
- Kim J, Kim Y, Kim E. Initial negative surgical margins versus revised negative surgical margins in patients who underwent surgery without adjuvant therapy for early-stage oral tongue squamous cell carcinoma. Oral Oncol. 2024;159. doi:https://doi.org/10.1016/j.oraloncology.2024.107046
- Kerawala C, Ong T. Relocating the site of frozen sections--is there room for improvement?. Head Neck. 2001;23:230-232. doi:https://doi.org/10.1002/1097-0347
- Scholl P, Byers R, Batsakis J. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue. Prognostic and therapeutic implications. Am J Surg. 1986;152:354-360. doi:https://doi.org/10.1016/0002-9610
- Johnson R, Sigman J, Funk G. Quantification of surgical margin shrinkage in the oral cavity. Head Neck. 1997;19:281-286. doi:https://doi.org/10.1002/(sici)1097-0347(199707)19:4<281::aid-hed6>3.0.co;2-x
- Mistry R, Qureshi S, Kumaran C. Post-resection mucosal margin shrinkage in oral cancer: quantification and significance. J Surg Oncol. 2005;91:131-133. doi:https://doi.org/10.1002/jso.20285
- Cheng A, Cox D, Schmidt B. Oral squamous cell carcinoma margin discrepancy after resection and pathologic processing. J Oral Maxillofac Surg. 2008;66:523-529. doi:https://doi.org/10.1016/j.joms.2007.08.040
- Pangare T, Waknis P, Bawane S. Effect of formalin fixation on surgical margins in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2017;75:1293-1298. doi:https://doi.org/10.1016/j.joms.2016.11.024
- Umstattd L, Mills J, Critchlow W. Shrinkage in oral squamous cell carcinoma: an analysis of tumor and margin measurements in vivo, post-resection, and post-formalin fixation. Am J Otolaryngol. 2017;38:660-662. doi:https://doi.org/10.1016/j.amjoto.2017.08.011
- Weinstock Y, Alava I, Dierks E. Pitfalls in determining head and neck surgical margins. Oral Maxillofac Surg Clin North Am. 2014;26:151-162. doi:https://doi.org/10.1016/j.coms.2014.01.003
- Michcik A, Jopek M, Pęksa R. Oral squamous cell carcinoma and what we lose during formalin fixation: an evaluation of changes in macroscopic surgical margins utilizing virtual three-dimensional imaging techniques with analysis based on 947 measurements. Biomedicines. 2024;12. doi:https://doi.org/10.3390/biomedicines12122805
- Shah A. Postoperative pathologic assessment of surgical margins in oral cancer: a contemporary review. J Oral Maxillofac Pathol. 2018;22:78-85. doi:https://doi.org/10.4103/jomfp.JOMFP_185_16
- Abbey L, Kaugars G, Gunsolley J. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:188-191. doi:https://doi.org/10.1016/s1079-2104(05)80201-x
- Tirelli G, Zacchigna S, Boscolo Nata F. Will the mininvasive approach challenge the old paradigms in oral cancer surgery?. Eur Arch Otorhinolaryngol. 2017;274:1279-1289. doi:https://doi.org/10.1007/s00405-016-4221-0
- Burns C, Gorina Faz M. An analysis of tumor margin shrinkage in the surgical resection of squamous cell carcinoma of the oral cavity. Cureus. 2021;13. doi:https://doi.org/10.7759/cureus.15329
- Sarode S, Sarode G. A novel “microscopic method” of shrinkage calculation in the pursuance of shrinkage based histopathological guidelines for interpretation of surgical margins. Oral Oncol. 2012;48:E15-E16. doi:https://doi.org/10.1016/j.oraloncology.2012.01.011
- Sengupta N, Sarode G, Sarode S. Optimum surgical margins in squamous cell carcinoma of the oral tongue: does shrinkage matters?. Oral Oncol. 2021;113. doi:https://doi.org/10.1016/j.oraloncology.2020.105052
- Ebrahimi A, Murali R, Gao K. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer. 2011;117:4460-4467. doi:https://doi.org/10.1002/cncr.26032
- Li C, Lin J, Men Y. Does medullary versus cortical invasion of the mandible affect prognosis in patients with oral squamous cell carcinoma?. J Oral Maxillofac Surg. 2017;75:403-415. doi:https://doi.org/10.1016/j.joms.2016.08.005
- Fried D, Mullins B, Weissler M. Prognostic significance of bone invasion for oral cavity squamous cell carcinoma considered T1/T2 by American joint committee on cancer size criteria: significance of bone invasion. Head Neck. 2014;36:776-781. doi:https://doi.org/10.1002/hed.23367
- Nieberler M, Häusler P, Drecoll E. Evaluation of intraoperative cytological assessment of bone SM in patients with oral squamous cell carcinoma. Cancer Cytopathol. 2014;122:646-656. doi:https://doi.org/10.1002/cncy.21428
- Nieberler M, Häußler P, Kesting M. Intraoperative cell isolation for a cytological assessment of bone SM in patients with head and neck cancer. Br J Oral Maxillofac Surg. 2017;55:510-516. doi:https://doi.org/10.1016/j.bjoms.2017.02.006
- Nieberler M, Häußler P, Kesting M. Clinical impact of intraoperative cytological assessment of bone surgical margins in patients with head and neck carcinoma. Ann Surg Oncol. 2016;23:3579-3586. doi:https://doi.org/10.1245/s10434-016-5208-1
- Saturno M, Brandwein-Weber M, Greenberg L. Utilizing 3D head and neck specimen scanning for intraoperative margin discussions: proof of concept of our novel approach. Head Neck. 2023;45:10-21. doi:https://doi.org/10.1002/hed.27171
- Sharif K, Lewis J, Ely K. The computer-aided design margin: ex vivo 3D specimen mapping to improve communication between surgeons and pathologists. Head Neck. 2023;45:22-31. doi:https://doi.org/10.1002/hed.27201
- Van Houten V, Leemans C, Kummer J. Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients. Clin Cancer Res. 2004;10:3614-3620. doi:https://doi.org/10.1158/1078-0432.CCR-03-0631
- Westra W, Sidransky D. Phenotypic and genotypic disparity in premalignant lesions: of calm water and crocodiles. J Natl Cancer Inst. 1998;90:1500-1501. doi:https://doi.org/10.1093/jnci/90.20.1500
- Leemans C, Tiwari R, Nauta J. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73:187-190. doi:https://doi.org/10.1002/1097-0142
- De Carvalho A, Kowalski L, Campos A. Clinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral Oncol. 2012;48:240-248. doi:https://doi.org/10.1016/j.oraloncology.2011.10.018
- Van Schaik J, Van Der Vegt B, Slagter-Menkema L. Identification of new head and neck squamous cell carcinoma molecular imaging targets. Oral Oncol. 2024;151. doi:https://doi.org/10.1016/j.oraloncology.2024.106736
- Lauwerends L, Zweedijk B, Galema H. Tumor marker expression in head and neck malignancies to identify potential targets for intraoperative molecular near-infrared imaging. Mol Diagn Ther. 2024;28:811-820. doi:https://doi.org/10.1007/s40291-024-00742-w
- Brennan J, Mao L, Hruban R. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435. doi:https://doi.org/10.1056/NEJM199502163320704
- Babji D, Nayak R, Bhat K. Comparative evaluation of immunohistochemical expression of p16 with p16 microsatellite marker by PCR in surgical margins of oral squamous cell carcinoma. Indian J Otolaryngol Head Neck Surg. 2019;71:716-723. doi:https://doi.org/10.1007/s12070-018-1517-y
- Braakhuis B, Bloemena E, Leemans C. Molecular analysis of surgical margins in head and neck cancer: more than a marginal issue. Oral Oncol. 2010;46:485-491. doi:https://doi.org/10.1016/j.oraloncology.2010.01.019
- Baldan F, Gnan C, Lazarevic M. Somatic genomic imbalances in ‘tumor-free’ surgical margins of oral cancer. Int J Oral Maxillofac Surg. 2023;52:831-838. doi:https://doi.org/10.1016/j.ijom.2022.12.008
- Nathan C, Franklin S, Abreo F. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J Clin Oncol. 1999;17:2909-2914. doi:https://doi.org/10.1200/JCO.1999.17.9.2909
- Nathan C, Sanders K, Abreo F. Correlation of p53 and the proto-oncogene eIF4E in larynx cancers: prognostic implications. Cancer Res. 2000;60:3599-3604.
- Partridge M, Li S, Pateromichelakis S. Detection of minimal residual cancer to investigate why oral tumors recur despite seemingly adequate treatment. Clin Cancer Res. 2000;6:2718-2725.
- Sardi I, Franchi A, Ferriero G. Prediction of recurrence by microsatellite analysis in head and neck cancer. Genes Chromosomes Cancer. 2000;29:201-206. doi:https://doi.org/10.1002/1098-2264(2000)9999:9999<::aid-gcc1031>3.0.co;2-x
- Nathan C, Amirghahri N, Rice C. Molecular analysis of surgical margins in head and neck squamous cell carcinoma patients. Laryngoscope. 2002;112:2129-2140. doi:https://doi.org/10.1097/00005537-200212000-00003
- Temam S, Casiraghi O, Lahaye J. Tetranucleotide microsatellite instability in surgical margins for prediction of local recurrence of head and neck squamous cell carcinoma. Clin Cancer Res. 2004;10:4022-4028. doi:https://doi.org/10.1158/1078-0432.CCR-04-0199
- Handschel J, Oz D, Pomjanski N. Additional use of DNA-image cytometry improves the assessment of resection margins. J Oral Pathol Med. 2007;36:472-475. doi:https://doi.org/10.1111/j.1600-0714.2007.00564.x
- Huang X, Pateromichelakis S, Hills A. p53 mutations in deep tissues are more strongly associated with recurrence than mutation-positive mucosal margins. Clin Cancer Res. 2007;13:6099-6106. doi:https://doi.org/10.1158/1078-0432-CCR-07-1369
- Tan H, Saulnier P, Auperin A. Quantitative methylation analyses of resection margins predict local recurrences and disease-specific deaths in patients with head and neck squamous cell carcinomas. Br J Cancer. 2008;99:357-363. doi:https://doi.org/10.1038/sj.bjc.6604478
- Zhao H, Ren J, Zhuo X. Prognostic significance of Survivin and CD44v6 in laryngeal cancer surgical margins. J Cancer Res Clin Oncol. 2008;134:1051-1058. doi:https://doi.org/10.1007/s00432-008-0391-5
- Bergshoeff V, Hopman A, Zwijnenberg I. Chromosome instability in resection margins predicts recurrence of oral squamous cell carcinoma. J Pathol. 2008;215:347-348. doi:https://doi.org/10.1002/path.2349
- Simon R, Paik S, Hayes D. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446-1452. doi:https://doi.org/10.1093/jnci/djp335
- Schaaij-Visser T, Graveland A, Gauci S. Differential proteomics identifies protein biomarkers that predict local relapse of head and neck squamous cell carcinomas. Clin Cancer Res. 2009;15:7666-7675. doi:https://doi.org/10.1158/1078-0432.CCR-09-2134
- Sinha P, Bahadur S, Thakar A. Significance of promoter hypermethylation of p16 gene for margin assessment in carcinoma tongue. Head Neck. 2009;31:1423-1430. doi:https://doi.org/10.1002/hed.21122
- Poeta M, Manola J, Goldenberg D. The ligamp TP53 assay for detection of minimal residual disease in head and neck squamous cell carcinoma surgical margins. Clin Cancer Res. 2009;15:7658-7665. doi:https://doi.org/10.1158/1078-0432.CCR-09-1433
- Graveland A, Golusinski P, Buijze M. Loss of heterozygosity at 9p and p53 immunopositivity in surgical margins predict local relapse in head and neck squamous cell carcinoma. Int J Cancer. 2011;128:1852-1859. doi:https://doi.org/10.1002/ijc.25523
- Pena Murillo C, Huang X, Hills A. The utility of molecular diagnostics to predict recurrence of head and neck carcinoma. Br J Cancer. 2012;107:1138-1143. doi:https://doi.org/10.1038/bjc.2012.213
- Roh J, Westra W, Califano J. Tissue imprint for molecular mapping of deep surgical margins in patients with head and neck squamous cell carcinoma. Head Neck. 2012;34:1529-1536. doi:https://doi.org/10.1002/hed.21982
- Ogbureke K, Weinberger P, Looney S. Expressions of matrix metalloproteinase-9 (MMP-9), dentin sialophosphoprotein (DSPP), and osteopontin (OPN) at histologically negative surgical margins may predict recurrence of oral squamous cell carcinoma. Oncotarget. 2012;3:286-298. doi:https://doi.org/10.18632/oncotarget.373
- Sakashita T, Homma A, Suzuki S. Prognostic value of cyclin D1 expression in tumor-free surgical margins in head and neck squamous cell carcinomas. Acta Otolaryngol. 2013;133:984-991. doi:https://doi.org/10.3109/00016489.2013.795287
- Hayashi M, Wu G, Roh J. Correlation of gene methylation in surgical margin imprints with locoregional recurrence in head and neck squamous cell carcinoma. Cancer. 2015;121:1957-1965. doi:https://doi.org/10.1002/cncr.29303
- Lin J, Wang C, Jiang R. Impact of microsatellite alteration in surgical margins on local recurrence in oral cavity cancer patients. Eur Arch Otorhinolaryngol. 2017;274:431-439. doi:https://doi.org/10.1007/s00405-016-4215-y
- Singh J, Jayaraj R, Baxi S. Immunohistochemical expression levels of p53 and eIF4E markers in histologically negative surgical margins, and their association with the clinical outcome of patients with head and neck squamous cell carcinoma. Mol Clin Oncol. 2016;4:166-172. doi:https://doi.org/10.3892/mco.2015.689
- Wang X, Chen S, Chen X. Tumor-related markers in histologically normal margins correlate with locally recurrent oral squamous cell carcinoma: a retrospective study. J Oral Pathol Med. 2016;45:83-88. doi:https://doi.org/10.1111/jop.12334
- Pierssens D, Borgemeester M, van der Heijden S. Chromosome instability in tumor resection margins of primary OSCC is a predictor of local recurrence. Oral Oncol. 2017;66:14-21. doi:https://doi.org/10.1016/j.oraloncology.2016.12.029
- Roy S, Kar M, Roy S. Role of β-catenin in cisplatin resistance, relapse and prognosis of head and neck squamous cell carcinoma. Cell Oncol. 2018;41:185-200. doi:https://doi.org/10.1007/s13402-017-0365-1
- Liu S, Wang C, Jiang R. Genetic analysis of surgical margins in oral cavity cancer. Br J Surg. 2018;105:E142-E149. doi:https://doi.org/10.1002/bjs.10693
- Yang X, Ding L, Fu Y. p53-positive expression in dysplastic surgical margins is a predictor of tumor recurrence in patients with early oral squamous cell carcinoma. Cancer Manag Res. 2019;11:1465-1472. doi:https://doi.org/10.2147/CMAR.S192500
- Holmes B, von Eyben R, Attardi L. Pilot study of loss of the p53/p63 target gene PERP at the surgical margin as a potential predictor of local relapse in head and neck squamous cell carcinoma. Head Neck. 2020;42:3188-3196. doi:https://doi.org/10.1002/hed.26358
- Govindaraj P, Kallarakkal T, Mohd Zain R. Expression of Ki-67, Cornulin and ISG15 in non-involved mucosal surgical margins as predictive markers for relapse in oral squamous cell carcinoma (OSCC). PLoS One. 2021;16. doi:https://doi.org/10.1371/journal.pone.0261575
- Kamat M, Puranik R, Das Rai A. Assessing immuno-expression of p53 protein and TP 53 gene amplification in histologically negative surgical margins of oral squamous cell carcinoma patients and normal oral mucosa. Clin Oral Investig. 2022;26:6235-6243. doi:https://doi.org/10.1007/s00784-022-04574-y
- Goldenberg D, Harden S, Masayesva B. Intraoperative molecular margin analysis in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:39-44. doi:https://doi.org/10.1001/archotol.130.1.39
- Hayashi M, Guerrero-Preston R, Sidransky D. Paired box 5 methylation detection by droplet digital PCR for ultra-sensitive deep surgical margin analysis of head and neck squamous cell carcinoma. Cancer Prev Res. 2015;8:1017-1026. doi:https://doi.org/10.1158/1940-6207.CAPR-15-0180
- Saidak Z, Pascual C, Bouaoud J. A three-gene expression signature associated with positive SM in tongue squamous cell carcinomas: predicting surgical resectability from tumor biology?. Oral Oncol. 2019;94:115-120. doi:https://doi.org/10.1016/j.oraloncology.2019.05.020
- Ganci F, Allegretti M, Frascolla C. Combined TP53 status in tumor-free surgical margins and circulating microRNA profiling predicts the risk of locoregional recurrence in head and neck cancer. Biomark Res. 2024;12. doi:https://doi.org/10.1186/s40364-024-00576-y
- Xu Y, Deng X, Sun Y. Optical imaging in the diagnosis of OPMDs malignant transformation. J Dent Res. 2022;101:749-758. doi:https://doi.org/10.1177/00220345211072477
- Wu C, Gleysteen J, Teraphongphom N. In-vivo optical imaging in head and neck oncology: basic principles, clinical applications and future directions. Int J Oral Sci. 2018;10. doi:https://doi.org/10.1038/s41368-018-0011-4
- Wong Kee Song L, Adler D, Conway J. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581-589. doi:https://doi.org/10.1016/j.gie.2008.01.013
- Yagishita A, Fujii S, Yano T. Endoscopic findings using narrow-band imaging to distinguish between basal cell hyperplasia and carcinoma of the pharynx. Cancer Sci. 2014;105:857-861. doi:https://doi.org/10.1111/cas.12440
- Tirelli G, Piovesana M, Gatto A. Narrow band imaging in the intra-operative definition of surgical margins in oral cavity and oropharyngeal cancer. Oral Oncol. 2015;51:908-913. doi:https://doi.org/10.1016/j.oraloncology.2015.07.005
- De Wit J, Van Schaik J, Voskuil F. Comparison of narrow band and fluorescence molecular imaging to improve intraoperative tumor margin assessment in oral cancer surgery. Oral Oncol. 2022;134. doi:https://doi.org/10.1016/j.oraloncology.2022.106099
- Han R, Lin N, Huang J. Diagnostic accuracy of Raman spectroscopy in oral squamous cell carcinoma. Front Oncol. 2022;12. doi:https://doi.org/10.3389/fonc.2022.925032
- Ibrahim O, Toner M, Flint S. The potential of Raman spectroscopy in the diagnosis of dysplastic and malignant oral lesions. Cancers. 2021;13. doi:https://doi.org/10.3390/cancers13040619
- Cals F, Koljenović S, Hardillo J. Development and validation of Raman spectroscopic classification models to discriminate tongue squamous cell carcinoma from non-tumorous tissue. Oral Oncol. 2016;60:41-47. doi:https://doi.org/10.1016/j.oraloncology.2016.06.012
- Yan B, Wen Z, Xue L. GenAI synthesis of histopathological images from Raman imaging for intraoperative tongue squamous cell carcinoma assessment. Int J Oral Sci. 2025;17. doi:https://doi.org/10.1038/s41368-025-00346-y
- Fabelo H, Halicek M, Ortega S. Medical Imaging 2019: Image-Guided Procedures, Robotic Interventions, and Modeling. (Fei B, Linte C, eds.). SPIE; 2019. doi:https://doi.org/10.1117/12.2512569
- Boehm F, Alperovich A, Schwamborn C. Enhancing surgical precision in squamous cell carcinoma of the head and neck: hyperspectral imaging and artificial intelligence for improved margin assessment in an ex vivo setting. Spectrochim Acta A Mol Biomol Spectrosc. 2025;332. doi:https://doi.org/10.1016/j.saa.2025.125817
- Karnatz N, Möllmann H, Wilkat M. Advances and innovations in ablative head and neck oncologic surgery using mixed reality technologies in personalized medicine. J Clin Med. 2022;11. doi:https://doi.org/10.3390/jcm11164767
- Catanzaro S, Copelli C, Manfuso A. Intraoperative navigation in complex head and neck resections: indications and limits. Int J Comput Assist Radiol Surg. 2017;12:881-887. doi:https://doi.org/10.1007/s11548-016-1486-0
- Al-Sabahi M, Jamali O, Shindy M. Aesthetic reconstruction of onco-surgical mandibular defects using free fibular flap with and without CAD/CAM customized osteotomy guide: a randomized controlled clinical trial. BMC Cancer. 2022;22. doi:https://doi.org/10.1186/s12885-022-10322-y
- Nyirjesy S, Heller M, von Windheim N. The role of computer aided design/computer assisted manufacturing (CAD/CAM) and 3-dimensional printing in head and neck oncologic surgery: a review and future directions. Oral Oncol. 2022;132. doi:https://doi.org/10.1016/j.oraloncology.2022.105976
- Ceccariglia F, Cercenelli L, Badiali G. Application of augmented reality to maxillary resections: a three-dimensional approach to maxillofacial oncologic surgery. JPM. 2022;12. doi:https://doi.org/10.3390/jpm12122047
- Allegra E, Bianco M, Mignogna C. Early glottic cancer treated by transoral laser surgery using toluidine blue for the definition of the surgical margins: a pilot study. Medicina. 2020;56. doi:https://doi.org/10.3390/medicina56070334
- Zhou J, Yang F, Jiang G. Applications of indocyanine green based near-infrared fluorescence imaging in thoracic surgery. J Thorac Dis. 2016;8:S738-S743. doi:https://doi.org/10.21037/jtd.2016.09.49
- Scott-Wittenborn N, Jackson R. Intraoperative imaging during minimally invasive transoral robotic surgery using near-infrared light. Am J Otolaryngol. 2018;39:220-222. doi:https://doi.org/10.1016/j.amjoto.2017.09.001
- Hadjipanayis C, Stummer W. 5-ALA and FDA approval for glioma surgery. J Neurooncol. 2019;141:479-486. doi:https://doi.org/10.1007/s11060-019-03098-y
- Filip P, Lerner D, Kominsky E. 5-Aminolevulinic acid fluorescence-guided surgery in head and neck squamous cell carcinoma. Laryngoscope. 2024;134:741-748. doi:https://doi.org/10.1002/lary.30910
- van Keulen S, Nishio N, Fakurnejad S. The clinical application of fluorescence-guided surgery in head and neck cancer. J Nucl Med. 2019;60:758-763. doi:https://doi.org/10.2967/jnumed.118.222810
- Srinivasan A, Kaminskaite V, Winter S. The use of fluorescent markers to detect and delineate head and neck cancer: a scoping review. Clin Otolaryngol. 2025;50:220-240. doi:https://doi.org/10.1111/coa.14263
- Shavlokhova V, Flechtenmacher C, Sandhu S. Detection of oral squamous cell carcinoma with ex vivo fluorescence confocal microscopy: sensitivity and specificity compared to histopathology. J Biophotonics. 2020;13. doi:https://doi.org/10.1002/jbio.202000100
- Sethi S, Ju X, Logan R. Diagnostic accuracy of confocal laser endomicroscopy for the diagnosis of oral squamous cell carcinoma: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18. doi:https://doi.org/10.3390/ijerph182312390
- Zanoni D, Demétrio De Souza França P, Valero C. A prospective double-blinded comparison of reflectance confocal microscopy with conventional histopathology for in vivo assessment in oral cancer. Clin Cancer Res. 2024;30:2486-2496. doi:https://doi.org/10.1158/1078-0432.CCR-23-1361
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1353 times
- PDF downloaded - 317 times




