Head and neck
Vol. 45: 111TH CONGRESS SIOECHCF - OFFICIAL REPORT 2025
Margins in oncologic nasopharyngeal surgery: a systematic review with meta-analysis
Abstract
Objective. Nasopharyngeal malignancies are rare heterogenous histologies (nasopharyngeal carcinoma [NPC], minor salivary glands carcinomas, and low-grade papillary nasopharyngeal adenocarcinoma) and a significant proportion of patients experience loco-regional recurrence after primary treatment. Resection margin status is a key prognostic factor that influences recurrence and survival, although definitions and criteria for negative, close, and positive margins remain inconsistent. This systematic review with meta-analysis aimed to summarise the existing definitions of resection margins in the literature and evaluate their impact on clinical outcomes in patients undergoing nasopharyngectomy with a specific focus on NPC.
Methods. A systematic literature review was conducted according to PRISMA guidelines. Electronic databases (Scopus, PubMed, and Web of Science) were searched up to November 2024. Studies reporting on surgical margins and survival outcomes in patients with NPC treated with endoscopic or open nasopharyngectomy were included. Pooled odds ratios (OR) for overall survival (OS), diseasespecific survival (DSS), and disease-free survival (DFS) were calculated using a random-effects model.
Results. A total of 45 studies met the inclusion criteria, with 12 included in the meta-analysis. Positive surgical margins were associated with worse 5-year DFS (OR 2.21, 95% CI 1.55-3.14, p < 0.001), while no significant impact was observed on 3-year DFS (OR 2.3, p = 0.239), 3-year OS (OR 2, p = 0.167), 5-year OS (OR 2.98, p = 0.115), 3-year DSS (OR 1.25, p = 0.761), or 5-year DSS (OR 2.57, p = 0.265). Margin positivity rates were 16.9% for endoscopically-treated NPC, 20.6% for open-surgery NPC, and 20.6% for mixed histology, with no significant difference between surgical approaches (p = 0.995).
Conclusions. Positive resection margins significantly impact DFS in recurrent NPC. Standardised margin definitions are needed to improve prognostication and guide decisions on adjuvant therapy.
Introduction
Nasopharyngeal tumours comprise 0.7% of all cancers with 80,000 new cases annually worldwide 1. Most of these tumours are nasopharyngeal carcinoma (NPC), even if additional histologic subtypes exist, including minor salivary gland carcinoma (MiSGC) and low-grade papillary adenocarcinoma (LGPA) 2. Due to its radiosensitivity, the primary treatment for NPC is represented by non-surgical treatment including radiotherapy (RT), with induction and/or concomitant chemotherapy based on tumour stage 3. However, despite advances in treatment 4, approximately 7-13% of patients with NPC present residual loco-regional disease, and 10-40% develop a relapse within 2 years from initial therapy 5, thus making residual/recurrent NPC (rNPC) a non-negligible occurrence. Management of rNPC typically involves re-RT or salvage surgery 6, with the latter demonstrating superior loco-regional control and overall survival (OS) alongside a reduced incidence of severe complications such as radionecrosis and massive haemorrhage 3.
Unlike NPC, where RT plays a central role in primary treatment, MiSGC and LGPA of the nasopharynx respond poorly to photon-based RT; as a result, surgery is considered a fundamental step in the treatment of such rare nasopharyngeal cancers 7.
However, the complex anatomy of the skull base and the proximity with relevant neurovascular structures pose significant challenges in surgical management, requiring a careful preoperative study to avoid major neurovascular complications 8. Thus, resection margin status following nasopharyngectomy is a critical factor, with prognostic impact on both local control and OS 9. Nevertheless, a standardised definition of negative, close, and positive margins is still lacking: current definitions commonly describe margins as “negative” when the tumour-free distance exceeds 5 mm and “positive” when tumour is present at the resection edge 10. The classification of margins below 5 mm remains inconsistent across studies. While some explicitly define this range as “close,” others do not address it, leading to variability in interpretation and clinical decision-making 11. In case of positive margins, adjuvant re-RT is often indicated for previously treated NPC to mitigate the adverse prognostic impact and enhance local control; similarly, adjuvant RT is often considered for positive resection margins in MiSGC. Conversely, for close or negative margins, the need for adjuvant therapy depends on factors such as tumour stage and risk of microscopic residual disease, leaving the indication to adjuvant therapies for multidisciplinary debate 4.
The primary aim of this systematic review is to highlight the definitions and criteria for resection margins reported in the literature and assess the impact of margin status on clinical outcomes following nasopharyngectomy for malignant tumours, with a specific focus on rNPC.
Materials and methods
Protocol registration
The protocol of this systematic review and meta-analysis was registered on PROSPERO 12, an international database of prospectively registered systematic reviews in health and social care (Center for Reviews and Dissemination, University of York, York, UK), in December 2024 with registry number CRD42024617286.
Search strategy
A systematic review of the literature was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations 13. The electronic databases Scopus, PubMed, and Web of Science were searched from database inception to 21st November 2024.
The inclusion criteria for selection of studies were chosen according to the PICOS tool: Patients (P), adults affected by NPC; Intervention (I), endoscopic or open surgical approach; Comparator (C), none; Outcomes (O), OS as primary outcome, local control (LC), disease-free survival (DFS), and disease-specific survival (DSS) as secondary outcomes; Study design (S), retrospective and prospective cohort studies.
A combination of MeSH terms and free-text words was utilised to search for: “rhinopharynx” or “rhinopharyngeal” or “nasopharynx” or “nasopharyngeal” and “carcinoma” and “surgical margin” or “margin status” (Supplement material A). The reference lists of all the included articles were thoroughly screened to find other relevant studies. References were exported to Zotero bibliography manager (v6.0.10, Center for History and New Media, George Mason University, Fairfax, VA, USA). After removal of duplicates, 2 reviewers (AD and MF) independently screened all titles and abstracts and then evaluated the full texts of the eligible articles based on the inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus. No ethics approval or informed consent were required for this study since all data were obtained from published literature.
Selection criteria
Studies were deemed eligible when the following inclusion criteria were met: (i) confirmed histopathological diagnosis of nasopharyngeal malignancy; (ii) patients treated with open or endoscopic approach for nasopharyngeal malignancy; (iii) margin status clearly assessed; (iv) studies reporting at least 5 cases. Studies were considered eligible for the meta-analysis if event rates were available for the positive and negative surgical margins cohorts.
Exclusion criteria were as follows: (i) inaccessibility to full text; (ii) overlapping cohorts or articles with data redundancy; (iii) lack of relevant clinicopathological data in terms of margin status and outcome data; (iv) non-segregable data/inseparable cases between surgical and non-surgical cohorts; (v) non-original articles (i.e. letters, congress abstract, editorials, or book chapters); (vi) animal model studies; (vii) non-English language studies.
Data extraction and quality assessment
Extracted data were collected in an electronic database, including first author, year of publication, country of origin, patient age and gender, TNM staging, treatment modality, margin status, follow-up, and outcomes. The studies were categorised by histology into NPC, subdivided according to the surgical approach in endoscopically-treated NPC (eNPC) or NPC treated with an open approach (oNPC), and mixed histologies, which included non-segregable histology, and minor salivary gland malignancy studies. For consistency, 2 mixed surgery cohort studies (endoscopic and open) 10,14 addressing NPC were included in the open surgery group because the cohort had a higher proportion of open approaches compared to endoscopic ones. When specific data on whole-population survival and survival based on surgical margins were unavailable, these outcomes were extracted and inferred from raw data provided in the text of individual articles.
The quality of studies eligible for inclusion was categorised as poor, fair or good, in agreement with the National Institutes of Health quality assessment tool for Observational Cohorts and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed on 2nd December 2024)15. Two reviewers (AD and MF) independently evaluated the papers, and any disagreement was resolved by discussion.
Statistical analysis
The software RStudio (version 4.3.1, https://www.rstudio.com/) was employed for statistical analysis. We calculated event rates for the following 3- and 5-year outcomes: OS, considering death from any cause as an event; DSS, considering disease-specific death as an event; DFS, considering disease recurrence or death from any cause as an event. When survival data were not reported, they were extracted from Kaplan-Meier curves using the Engauge Digitizer software.
The odds ratio (OR) of the event rates was calculated for histologies with at least 3 studies included, comparing positive and negative margins groups. A pooled OR with a 95% confidence interval (CI) was calculated using the DerSimonian and Laird random-effects model 16. The p value was derived using a Z-test based on the pooled OR and its standard error. A p value < 0.05 was considered statistically significant.
To measure overall heterogeneity across the included cohorts, we calculated the I2 statistic 17. We assessed potential publication bias by visual inspection of the symmetry of funnel plots and with the Egger regression asymmetry test 18.
Results
Search results and quality assessment
A total of 3,639 titles were retrieved from the literature search. After removing duplicates and excluding 2,321 records, 87 articles relevant to the topic were examined. One study was unavailable for retrieval. Following the application of inclusion and exclusion criteria, 41 studies were excluded, leaving 45 for qualitative synthesis. Of these, 27 focused on eNPC 3,19-44, 11 on oNPC 10,14,45-53, and 7 on mixed histology 7,8,54-58. Twelve studies were included in the quantitative analysis based on the availability of event rate data stratified by margin status. Of these, 7 focused on locally recurrent eNPC 21,22,25,31,32,34,39, and 5 on locally recurrent oNPC 10,46,47,49,53. A detailed PRISMA flowchart of the search process is presented in Figure 1.
According to the National Institutes of Health Quality Assessment Tool for Observational Cohorts and Cross-Sectional Studies 15, 18 studies (40%) were deemed of good quality, 23 (51.1%) of fair quality, and 4 (8.9%) of poor quality due to lack of relevant clinical details (Supplement material B). All studies included demonstrated adequate relevance to the subject of this systematic review. Only one randomised controlled trial 35 was identified. The remaining studies comprised prospective and retrospective case series. Publications spanned from 2000 to 2024, covering surgical cases from 1984 to 2022.
Systematic review of the literature for NPC
The total number of patients with NPC was 2,070, with 1,198 (57.9%) eNPC and 872 (42.1%) oNPC. The median ages were 50 years (range, 21-81) and 49 years (range, 24–85) for eNPC and oNPC, respectively. Twenty of the 27 eNPC studies (74.1%) involved patients from endemic regions, compared to 10 of the 11 oNPC studies (90.9%). The median time to recurrence after primary treatment was 27 months (range, 3-201) and 27.4 months (range, 2-280) for eNPC and oNPC, respectively. Median follow-up was 30.3 months (range, 1-139) for patients with eNPC and 38 months (range, 0.1-268) for those with oNPC.
Positive and negative surgical margins were identified in 201 (16.9%) and 991 (83.1%) cases for eNPC, and 175 (20.6%) and 674 (79.4%) for oNPC, respectively. Local recurrence/persistence after the last treatment was reported in 210 (17.5%) and 77 (8.8%) patients for eNPC and oNPC, respectively. General characteristics of the studies included are summarised in Table I.
Systematic review of the literature for mixed histology group
The mixed histology group spanned from adenoid cystic carcinoma 55, mucoepidermoid carcinoma 58, and LGPA 54 to mixed non-separable cohorts 7,8,56,57. The total number of patients was 475, with a median age of 47 years (range, 7-77). Upfront surgery was reported in 377 (79.4%) patients and salvage surgery in 98 (20.6%). Median follow-up was 41 months (range, 2-173). Positive and negative surgical margins were identified in 95 (20.6%) and 366 (79.4%) cases, respectively, with local recurrence or persistence after the last treatment reported in 19 (4%) patients.
Definition of margins
The definition of margins was provided in only 22 studies (48.9%). Among them, 2 (9%) used a 2 mm threshold to define clear margins 21,23, 8 (36.4%) based the definition of negative margins on a 5 mm cutoff 10,19,28,31,34,37,51,57, and 9 (41%) relied on analysis of frozen sections 3,7,8,14,22,24,48,50,52, without any metric definition. Additionally, 3 (13.6%) studies differentiated the definition of negative margins between superficial/mucosal margins (threshold ranging between 5-10 mm) and deep/basal margins (2-3 mm), the latter referring to the surface of the sphenoid bone and clivus 29,35,43.
Meta-analysis for NPC
Twelve studies met inclusion criteria for the meta-analysis. Positive surgical margins were identified in 147 (21.8%) patients, 57 (8.9%) in the eNPC group, and 90 (13.4%) in the oNPC group. Survival outcomes stratified for negative (R0) and positive margins (R1) are summarised in Table II. The rate of positive surgical margins did not differ significantly between endoscopic and open surgical approaches (p = 0.995). The comparison of primary outcomes for R0 and R1 cohorts is summarised in Table II.
The meta-analysis included 9 studies for the 3-year OS outcome and 5 for the 5-year OS outcome. The pooled 3-year OS was 72.7% for the R0 group and 60.1% for the R1 group, with a pooled OR of 2 (p = 0.167, Fig. 2A). The pooled 5-year OS was 62% for the R0 group and 26% for the R1 group; pooled OR was 2.98 (p = 0.115, Fig. 2B). DSS followed a similar trend, with pooled 3- and 5-year DSS rates of 72.9% and 61.9% in R0 patients, and 65.9% and 29.9% in R1 patients, respectively. The OR were 1.25 (p = 0.761, Fig. 3A) and 2.57 (p = 0.265, Fig. 3B). The pooled 3-year DFS was 64.2% and 47.2% for R0 and R1 patients, respectively, with a pooled OR of 2.3 (p = 0.239, Fig. 4A). The pooled 5-year DFS was 56.6% for the R0 group and 26.4% for the R1 group, with a pooled OR of 2.83 (p < 0.001, Fig. 4B).
The studies included demonstrated a moderate heterogeneity. The funnel plot (Fig. 5) was used to assess publication bias. Egger’s test for asymmetry showed no significant small-study effects, indicating no substantial publication bias for any of the outcomes (3-year OS, p = 0.669; 5-year OS, p = 0.276; 3-year DSS, p = 0.972; 5-year DSS, p = 0.615; 3-year DFS, p = 0.765; 5-year DFS, p = 0.848).
Discussion
Nasopharyngeal carcinoma
The present study confirms that negative margins have a protective effect on DFS, although their impact on OS and DSS did not reach statistical significance. To the best of our knowledge, this is the first study to assess the prognostic effect of margin status in a large cohort of rNPC. Overall, the rate of positive margins was 16.9% for eNPC and 20.6% for oNPC (reported ranges in the literature between 0% and 71.7%) 3,10,14,19-53. The OR for 3-year DFS was 2.3 (p = 0.239), while for 5-year DFS it was 2.83 (p < 0.001) indicating a significantly worse prognosis in the intermediate-to-long term. No significant association was found for 3-year OS (OR = 2, p = 0.167), 5-year OS (OR = 2.98, p = 0.115), 3-year DSS (OR = 1.25, p = 0.761) or 5-year DSS (OR = 2.57, p = 0.265). These results may be influenced by the presence of studies reporting an opposite trend, where patients with positive margins exhibited better outcomes 21,25,46. A plausible explanation is that these patients more frequently received adjuvant treatment, potentially mitigating the negative impact of margin positivity and confounding the prognostic effect of margins. However, this hypothesis could not be systematically verified through a pooled analysis due to the lack of data from the studies included. Nonetheless, these findings suggest that patients with positive margins might benefit from adjuvant treatment after surgery 59. However, the increased risk of RT-induced adverse events in patients receiving postoperative re-RT shortly after surgery should be weighted and personalised to each patient, as described by Chen et al. 23. One could hypothesise that the surgical approach (open vs. endoscopic) may influence margin status, as suggested by the meta-analysis of Na’ara et al. 60 that demonstrated significantly better survival outcomes with an endoscopic approach compared to open surgery (2-year OS 88% vs 60%, p = 0.039), with an even greater advantage in 5-year OS for patients with advanced-stage tumours (66% vs 12%, p = 0.009). In our series, we attempted to analyse differences in surgical margins between open and endoscopic approaches, but the results were not statistically significant (p = 0.995), leaving the influence of the surgical approach on margins open to debate 61.
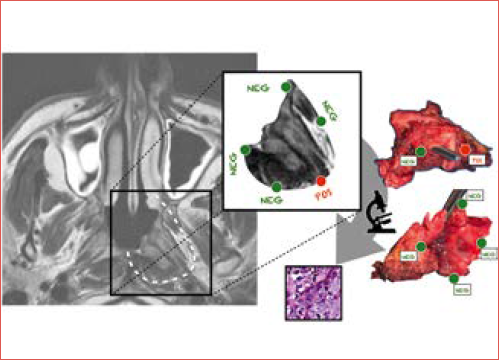
Pathological assessment of surgical margins remains the gold standard for determining complete tumour excision (Cover figure). However, at definitive histology, false positive margins may occur due to thermal artifacts, sampling errors, or fixation techniques 62. To enhance intraoperative margin delineation and evaluation, various techniques have been proposed, including intraoperative surgical navigation 63 and use of frozen sections 64. In this setting, real-time collaboration between surgeon and pathologist enables accurate assessment of margins and immediate additional resection, if necessary 65. However, this approach requires longer operative time, as well as the availability of an experienced pathologist.
Among the studies included in the present meta-analysis, 37 (82.2%) provided information regarding surgical margins, even if definition of margin status was clearly reported in only 22 (59.5%) articles; of these, 12 (54.5%) reported on survival outcomes stratified by margin status and were included in the meta-analysis.
Surgical margin definitions varied substantially, including thresholds of 2, 5, and 10 mm, or margins determined by intraoperative frozen section analysis only. This variability poses a challenge in establishing standardised prognostic cutoffs. A distinct approach was described in 3 studies that differentiated the definition of negative margins between superficial/mucosal margins (threshold ranging between 5-10 mm) and deep/basal margins (range between 2-3 mm), the latter referring to the surface of the sphenoid bone and clivus 29,35,43.
Neoadjuvant chemotherapy in rNPC
The role of neoadjuvant chemotherapy (CT) in rNPC remains a topic of debate, particularly regarding its impact on surgical margins. Several studies have suggested that neoadjuvant CT may downstage tumours, reduce tumour burden, and potentially facilitate complete resection with negative margins 66,67. However, neoadjuvant CT may also induce fibrosis and alter the tumour-stroma interface, thus making intraoperative and definitive pathological assessment of margins more complex 11. Additionally, the development of multifocal lesions after neoadjuvant CT increases the risk of false negative margins 68,69.
As a final remark, the prognostic significance of margin status following neoadjuvant CT is not well established. While negative margins are traditionally associated with better outcomes, post-neoadjuvant CT microscopic residual disease may have a different biological behaviour than de novo tumour infiltration. Some studies suggest that persistent tumour viability, rather than margin status alone, may be a stronger prognostic factor in patients undergoing surgery after neoadjuvant CT 11,70. This raises the question of whether traditional margin assessment criteria should be adjusted after neoadjuvant CT. Resection margins should be determined based on pre-neoadjuvant CT imaging, as using neoadjuvant CT to reduce resection volume is not advisable 11. Future studies should clarify if neoadjuvant systemic therapy affects interpretation of margins and whether margin status remains prognostically relevant in this setting.
Nasopharyngeal MiSGC and LGPA
MiSGC, such as adenoid cystic and mucoepidermoid carcinomas, as well as LGPA, are very rare histologies of the nasopharynx. Surgery constitutes a fundamental step in the treatment of resectable MiSGC, which almost invariably includes RT in spite of their variable radiosensitivity. On the other hand, surgery represents the treatment of choice for LGPA of the nasopharynx, given its radioresistance. Considering these different cancer types as a single group, the rate of positive margins was 20.6% in the present pooled series 7,8,54-58. Owing to the rarity of these lesions, we were unable to extract pooled results due to the lack of sufficient data in the current literature.
However, several key aspects regarding management of nasopharyngeal MiSGC have been previously established. Unlike NPC, which is highly radiosensitive, these tumours show a variable sensitivity to RT regardless of histopathologic grade. Consequently, achieving negative surgical margins is crucial, as adjuvant RT may have an unpredictable role in controlling residual disease. While 3 studies qualitatively suggested a protective effect of negative margins for the most common MiSGCs (i.e., adenoid cystic and mucoepidermoid carcinomas) 55 and LGPA 54, the lack of pooled data prevents definitive conclusions.
This review has several limitations. All studies included were retrospective, except for one, which was a prospective randomised trial 40. The heterogeneity and incomplete data in the literature poses challenges in conducting a comprehensive meta-analysis, particularly due to the lack of survival data stratified by margin status and staging, which limited assessment of outcomes. Furthermore, the small number of studies and insufficient data prevented a comparative analysis between EBV-related and unrelated NPC. Standardisation of data presentation in this field is essential, and some survival outcomes were extrapolated rather than obtained directly.
Conclusions
This study highlights the protective role of negative margins in nasopharyngectomy for rNPC, with a significant association between margin status and DFS. Although the impact on OS and DSS did not reach statistical significance for rNPC, the potential impact of margin status on prognosis warrants further investigation. Regarding MiSGCs of the nasopharynx, our findings emphasise the importance of achieving clean surgical margins given their acknowledged radioresistance. However, the limited data on minor salivary gland tumours prevented a pooled analysis, underscoring the need for further research to clarify the prognostic implications of margin status in these rare tumours. Both open and endoscopic nasopharyngectomy remain viable surgical approaches when performed by experienced surgeons. Enhancing intraoperative margin assessment through techniques such as use of frozen sections and real-time pathological evaluation may help in optimising surgical outcomes. Future prospective studies are needed to establish standardised margin thresholds in nasopharyngeal oncologic surgery.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
AD, PG, MF, PB: conceptualisation; AD, PG, MF: methodology; AD, PG: software; PC, PN, MTZ, MF, BP: validation; AD, PG: formal analysis; AD, MF: investigation; AD, PG, MF, PB: resources; AD, PG: data curation; AD, PG, AV: writing – original draft preparation; AD, PG, AV, ST, PC, PN, MTZ, MF, PB: writing – review and editing; PC, PN: visualisation; PC, PN: supervision. All authors have read and agreed to the published version of the manuscript.
Ethical consideration
Not applicable.
History
Received: March 24, 2025
Accepted: March 31, 2025
Figures and tables
Figure 1. PRISMA diagram summarising the electronic database search and inclusion/exclusion process of the review.
Figure 2. Forest plot for overall survival at 3- (A) and 5-years (B).
Figure 3. Forest plot for disease-specific survival at 3- (A) and 5-years (B).
Figure 4. Forest plot for disease-free survival at 3- (A) and 5-years (B).
Figure 5. Funnel plot of meta-analysis. Black dots identify each study’s characteristics in terms of standard error and standardised mean difference.
| First author | Year | Country | Period of enrollment | No. of patients | Median age (range) | Gender (M/F) | Endemic area | Histology | Surgical approach | Margin status (%) | Median FU months (range) | Local progression/recurrence (n) | OS (%) | DSS (%) | DFS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 19 | 2009 | China | 2004-2008 | 37 | 47 (27-71) | 27/10 | Yes | NPC | Endoscopic | R0 36 (97.3) R1 1 (0.7) | 24 (6-45) | 5 | 2y 84.2 | NA | NA |
| Rohaizam 20 | 2009 | Malaysia | 2007-2009 | 6 | 49.7 (37-61) | 3/3 | Yes | NPC | Endoscopic | R0 6(100) R1 0 (0) | 7.3 (3-14) | 0 | 1y 100 | NA | NA |
| Ko 21 | 2009 | Taiwan | 2004-2007 | 28 | 51 (31-70) | 21/7 | Yes | NPC | Endoscopic | R0 25 (89.3) R1 3 (10.7) | 16.2 (3-48) | 7 | 2y 59.4 | NA | 2y 57.6 |
| Ho 22 | 2012 | USA | 2005-2010 | 13 | 55.7 (35-81) | 9/4 | Yes | NPC | Endoscopic | R0 9 (69.3) R1 4 (30.7) | 24.2 (3.2-48.6) | 5 | 2y 100 | NA | 2y 69.2 |
| Chen 23 | 2013 | Taiwan | 2005-2010 | 33 | 50.5 (36-77) | 25/8 | Yes | NPC | Endoscopic | R0 30 (90.9) R1 3 (9.1) | 42.5 (8-93) | 10 | 5y 75.8 | NA | 2y 76.6 |
| Emanuelli 24 | 2014 | Italy | 2008-2011 | 8 | 59.1 (45-83) | 6/2 | No | NPC | Endoscopic | R0 8(100) R1 0 (0) | 26.9 (7-54) | 1 | 2y 100 | NA | 2y 88.9 |
| Hsu 25 | 2014 | Taiwan | 2009-2013 | 9 | 46.4 (32-63) | 6/3 | Yes | NPC | Endoscopic | R0 8 (88.9) R1 1 (11.1) | 24.9 (10-45) | 1 | 2y 100 | NA | 2y 80 |
| Weng 26 | 2017 | China | 2011-2013 | 36 | NA | 26/10 | Yes | NPC | Endoscopic | R0 33 (91.7) R1 3 (8.3) | 31 | 17 | 2y 69.23y 63.3 | NA | 2y 64.13y 61 |
| Vlantis 27 | 2017 | China | NA | 18 | 51.8 (26-79) | 11/7 | Yes | NPC | Endoscopic | R0 17 (94.4) R1 1 (5.6) | 22 | 1 | 2y 100 | NA | 2y 90 |
| Liu 3 | 2017 | China | 2005-2015 | 91 | 51 (28-80) | 71/20 | Yes | NPC | Endoscopic | R0 74 (81.3) R1 17 (18.7) | 23 (4-109) | 30 | 2y 62.53y 555y 38.3 | 5y 32 | 3y 555y 30.2 |
| Tang 28 | 2019 | Malaysia | 2013-2017 | 55 | 52.5 (21-69) | 44/11 | Yes | NPC | Endoscopic | R0 51 (92.7) R1 4 (7.3) | 18 (12-48) | 5 | 1y 98 | NA | NA |
| Liu 29 | 2019 | China | 2007-2017 | 10 | 47 (29-73) | 7/3 | Yes | NPC | Endoscopic | R0 10(100) R1 0 (0) | 59 | 0 | 3y 1005y 100 | NA | 2y 1003y 1005y 100 |
| Liu 30 | 2020 | China | 2016-2019 | 101 | 51 | 67/34 | Yes | NPC | Endoscopic | R0 85 (84.2) R1 16 (15.8) | 20 (5-44) | 25 | 2y 76.2 | NA | NA |
| Wong 31 | 2020 | Malaysia | 2003-2015 | 12 | NA | 7/5 | Yes | NPC | Endoscopic | R0 7 (58.3) R1 5 (41.7) | 44.8 (10-101) | 9 | 5y 50 | 5y 58.3 | 5y 25 |
| Tan 32 | 2020 | Malaysia | 2010-2018 | 7 | 57.6(39-78) | 6/1 | Yes | NPC | Endoscopic | R0 5 (71.4)R1 2 (28.6) | 30.28(4-48) | 3 | 2y 100 | NA | 2y 85.7 |
| Li 34 | 2021 | China | 2014-2019 | 120 | NA | 88/32 | Yes | NPC | Endoscopic | R0 85 (70.8)R1 35 (29.2) | 18 (2-81) | 12 | 2y 67.13y 605y 53 | NA | NA |
| Liu 35 | 2021 | China | 2011-2017 | 100 | 46 (38-55) | 69/31 | Yes | NPC | Endoscopic | R0 92(100)R1 4(4)NA 4(4) | 56 | 16 | 2y 89.93y 85.85y 73.8 | NA | 2y 81.83y 76.55y 59 |
| Thamboo 36 | 2021 | USA | 2000-2012 | 13 | 56.2 (35-80.8) | 9/4 | Yes | NPC | Endoscopic | R0 10 (76.9)R1 3 (23.1) | 74.3 (56.4-96) | 6 | 5y 84.6 | NA | 5y 53.9 |
| Wang 37 | 2022 | China | 2015-2020 | 37 | 48 (25-71) | 26/11 | Yes | NPC | Endoscopic | R0 36 (97.3)R1 1 (2.7) | 31 (5-53) | 10 | 2y 88.73y 63.5 | NA | NA |
| Iftikhar 38 | 2023 | UK | 2017-2021 | 6 | 43.7 (31-59) | 3-3 | No | NPC | Endoscopic | R0 6(100)R1 0 (0) | 36.5 | 3 | - | NA | 2y 66 |
| Li 33 | 2023 | China | 2009-2020 | 192 | NA | NA | Yes | NPC | Endoscopic | R0 148 (77.1)R1 44 (22.9) | 19 (1-118) | NA | 2y 70.63y 59.35y 49.7 | NA | NA |
| Rampinelli 39 | 2023 | Italy | 1998-2020 | 65 | NA | NA | No | NPC | Endoscopic | R0 52(80)R1 9 (13.8)NA 4 (6.2) | 29 (3-160) | NA | 5y 51.1 | NA | 5y 40.9 |
| Liu 40 | 2023 | China | 2013-2019 | 53 | 49 (39-55) | 45/8 | Yes | NPC | Endoscopic | R0 15 (28.3)R1 38 (71.7) | 30.4 | 18 | 2y 75.23y 68.45y 55 | 3y 80.95y 72.2 | NA |
| Xu 41 | 2023 | China | 2017-2021 | 30 | NA | 24/6 | Yes | NPC | Endoscopic | R0 30(100)R1 0 (0) | 37.8 (7-66) | 9 | 2y 82.5 | NA | 2y 37.3 |
| Zou 43 | 2024 | China | 2019-2020 | 56 | 47 (23-68) | 46/10 | Yes | NPC | Endoscopic | R0 56(100)R1 0 (0) | 44.3 (17.1-52.7) | 4 | 2y 96.43y 92.9 | NA | NA |
| Wu 42 | 2024 | China | 2015-2020 | 30 | 48 | 20/10 | Yes | NPC | Endoscopic | R0 28 (93.3)R1 2 (6.7) | 43 (2-80) | 7 | 2y 703y 62.9 | NA | NA |
| Valentini 44 | 2024 | Italy | 2003-2022 | 41 | 50 (31-81) | 28/13 | No | NPC | Endoscopic | R0 34 (82.9)R1 7 (17.1) | 57 (12-139) | 9 | 3y 765y 60.7 | 3y 82.75y 69 | 3y 52.75y 39.7 |
| King 45 | 2000 | China | 1986-1997 | 31 | 43 (25-66) | 27/4 | Yes | NPC | Open | R0 22 (70.9)R1 9 (29.1) | 29.3 (3-70) | 5 | 5y 47 | NA | 5y 42 |
| Shu 46 | 2000 | Taiwan | 1991-1997 | 28 | 53 (28-72) | 24/4 | Yes | NPC | Open | R0 21(75)R1 7(25) | 32.2 (0.1-93) | 7 | 2y 51.35y 36.6 | 5y 36.6 | 2y 48.35y 34.3 |
| Hao 47 | 2002 | Taiwan | 1993-1999 | 18 | 45.2 (32-59) | 13/5 | Yes | NPC | Open | R0 11 (61.1)R1 3 (16.7)Close 4 (22.2) | 32.1 (3-71) | 15 | 3y 575y 60 | 5y 61.5 | 5y 57.6 |
| Fee 48 | 2002 | USA | 1984-1999 | 37 | 49 (28-72) | 24/13 | NA | NPC | Open | R0 29 (78.4)R1 8 (21.6) | 64.8 (24-1020) | NA | 2y 755y 60 | NA | 5y 52 |
| Choi 49 | 2005 | South Korea | 1993-1999 | 11 | 47.5 (33-66) | 7/4 | Yes | NPC | Open | R0 9 (81.9)R1 2 (18.1) | 32.5 (9-56) | NA | 2y 72.73y 62.3 | 3y 62.3 | 2y 72.73y 51.9 |
| Hao 14 | 2008 | Taiwan | 1993-2006 | 53 | 50.6 (30-75) | 41/12 | Yes | NPC | Mixed | R0 36 (67.9)R1 17 (32.1) | 37.1 (5.1-142.2) | 20 | 5y 48.7 | NA | NA |
| Vlantis 50 | 2011 | China | 1987-2007 | 97 | 47.7 (26-70) | 72/25 | Yes | NPC | Open | R0 46 (47.4)R1 32(33)Close 18 (19.6) | NA (24-60) | NA | 5y 51.9 | NA | NA |
| Chan 51 | 2015 | China | 1990-2012 | 338 | 49.2 (24-81) | 262/76 | Yes | NPC | Open | R0 265 (78.4)R1 73 (21.6) | 34.3 (14-268) | NA | NA | NA | NA |
| Ng 52 | 2016 | Singapore | 2004-2011 | 20 | 49 (34-70) | 14/6 | Yes | NPC | Open | R0 20(100)R1 0 (0) | 60.4 | 7 | 5y 66.7 | 5y 70.2 | 5y 48.9 |
| Chan 10 | 2019 | China | 1990-2017 | 208 | 52.4 | 175/33 | Yes | NPC | Mixed | R0 189 (90.9)R1 19 (9.1) | 41.7 | 23 | 5y 51.3 | 5y 53.3 | NA |
| Tsang 53 | 2022 | China | 2010-2019 | 31 | 55 (29-85) | 20/11 | Yes | NPC | Open | R0 26 (83.9)R1 5 (16.1) | 38 (2.9-131) | NA | 2y 725y 55.7 | 3y 855y 69.1 | NA |
| Zhang 58 | 2010 | China | 1997-2009 | 13 | 45 (29-64) | 4/7 | NA | MEC | Open | R0 6 (46.2)R1 5 (38.5)NA 2 (15.3) | 43 (8-80) | NA | NA | NA | NA |
| Al-Sheibani 7 | 2011 | USA | 2002-2009 | 20 | 52 (36-63) | 12/8 | NA | Mixed | Endoscopic | R0 19(95)R1 1(5) | 33.6 (15-68) | NA | 2y 45 | NA | 2y 65 |
| Castelnuovo 8 | 2013 | Italy | 1997-2011 | 37 | NA | NA | NA | Mixed | Endoscopic | R0 33 (89.2)R1 3 (8.1)NA 1 (2.1) | 32.5 (2-173) | NA | 5y 75.1 | 5y 80.9 | 5y 58.1 |
| Lai 54 | 2021 | China | 2009-2019 | 28 | 41.5 (7-77) | 11/17 | NA | LGPA | Endoscopic | R0 28(100)R1 0 (0) | 54.7 (7-121) | 2 | 5y 100 | NA | NA |
| Finegersh 56 | 2022 | USA | 2004-2016 | 298 | 57.2 | 189/109 | NA | Mixed | Mixed | R0 207 (69.5)R1 77 (25.8)NA 14 (4.7) | NA | NA | 5y 57 | NA | NA |
| Chen 55 | 2024 | China | 2019-2021 | 12 | 40.7 (32-68) | 5/7 | NA | ACC | Endoscopic | R0 5 (41.7)R1 7 (58.3) | 24 (16-45) | 7 | NA | NA | NA |
| Zhao 57 | 2024 | China | 2005-2020 | 70 | 48 (21-74) | 41/29 | NA | Mixed | Mixed | R0 68 (97.1)R1 2 (2.9) | 39 | 10 | 3y 945y 88.7 | NA | 3y 91.15y 85.9 |
| ACC, adenoid cystic carcinoma; DFS, disease-free survival; DSS, disease-specific survival; LGPA, low-grade papillary adenocarcinoma; MEC, mucoepidermoid carcinoma;NA, not available; NPC, nasopharyngeal carcinoma; OS, overall survival | |||||||||||||||
| Outcome | Margin status | No. of patients | Pooled survival (%) | OR (95% CI) | I2 | p value |
|---|---|---|---|---|---|---|
| 3-yr OS | R0 | 246 | 72.7 | 2 (0.75-5.33) | 47% | 0.167 |
| R1 | 69 | 60.1 | ||||
| 5-yr OS | R0 | 110 | 62 | 2.98 (0.77-11.56) | 44% | 0.115 |
| R1 | 24 | 26 | ||||
| 3-yr DSS | R0 | 135 | 72.9 | 1.25 (0.3-5.23) | 48% | 0.761 |
| R1 | 29 | 65.9 | ||||
| 5-yr DSS | R0 | 84 | 61.9 | 2.57 (0.49-13.51) | 53% | 0.265 |
| R1 | 19 | 29.9 | ||||
| 3-yr DFS | R0 | 147 | 64.2 | 2.3 (0.57-9.22) | 57% | 0.239 |
| R1 | 36 | 47.2 | ||||
| 5-yr DFS | R0 | 356 | 56.6 | 2.83 (1.76-4.54) | 14% | < 0.001 |
| R1 | 97 | 26.4 | ||||
| CI, confidence interval; DFS, disease-free survival; DSS, disease-specific survival; OR, odds ratio; OS, overall survival; R0, negative margins; R1, microscopically positive margins. | ||||||
| PUBMED |
| (“rhinopharynx” OR “rhinopharyngeal” OR “nasopharynx” OR “nasopharyngeal”) AND (“neoplasm” OR “cancer” OR “pharynx neoplasm” OR “rhinopharynx neoplasm” OR “nasopharynx neoplasm” OR “carcinoma”[Mesh] OR “adenocarcinoma” OR “adenoid cyst carcinoma”) AND (“margin” OR “pathological” OR “surgical margin” OR “histology” OR “margin status” OR “Margin to depth ratio”) AND (“prognosis” OR “outcome” OR “survival” OR “impact” OR “failure pattern” OR “risk-tailored”) OR (“endoscopic nasopharyngectomy” OR “rhinopharyngectomy” OR “nasopharyngectomy”) |
| Filters: NO |
| SCOPUS |
| TITLE-ABS-KEY (“rhinopharynx” OR “nasopharynx”) AND TITLE-ABS-KEY (“neoplasm” OR “cancer” OR “pharynx neoplasm” OR “rhinopharynx neoplasm” OR “nasopharynx neoplasm” OR “carcinoma” OR “adenocarcinoma” OR “adenoid cyst carcinoma”) AND TITLE-ABS-KEY (“margin” OR “pathological” OR “surgical margin” OR “histology” OR “margin status” OR “Depth of invasion” OR “Margin to depth ratio”) AND (“prognosis” OR “outcome” OR “survival” OR “impact” OR “failure pattern” OR “risk-tailored”) OR (“endoscopic nasopharyngectomy” OR “rhinopharyngectomy” OR “nasopharyngectomy”) |
| Filters: NO |
| WEB OF SCIENCE |
| (“rhinopharynx” OR “rhinopharyngeal” OR “nasopharynx” OR “nasopharyngeal”) AND (“neoplasm” OR “cancer” OR “pharynx neoplasm” OR “rhinopharynx neoplasm” OR “nasopharynx neoplasm” OR “carcinoma” OR “adenocarcinoma” OR “adenoid cyst carcinoma”) AND (“margin” OR “pathological” OR “surgical margin” OR “histology” OR “margin status” OR “Depth of invasion” OR “Margin to depth ratio”) AND (“prognosis” OR “outcome” OR “survival” OR “impact” OR “failure pattern” OR “risk-tailored”) OR (“endoscopic nasopharyngectomy” OR “rhinopharyngectomy” OR “nasopharyngectomy”) |
| Filters: NO |
| First author | Year | Quality |
|---|---|---|
| King 45 | 2000 | Fair |
| Shu 46 | 2000 | Fair |
| Fee 48 | 2002 | Good |
| Choi 49 | 2005 | Fair |
| Hao 14 | 2008 | Fair |
| Chen 19 | 2009 | Fair |
| Rohaizam 20 | 2009 | Poor |
| Ko 21 | 2009 | Fair |
| Zhang 58 | 2010 | Poor |
| Vlantis 50 | 2011 | Good |
| Al-Sheibani 7 | 2011 | Fair |
| Ho 22 | 2012 | Fair |
| Chen 23 | 2013 | Fair |
| Castelnuovo 8 | 2013 | Good |
| Emanuelli 24 | 2014 | Good |
| Hsu 25 | 2014 | Fair |
| Chan 51 | 2015 | Good |
| Ng 52 | 2016 | Good |
| Weng 26 | 2017 | Good |
| Vlantis 27 | 2017 | Good |
| Liu 3 | 2017 | Good |
| Tang 28 | 2019 | Fair |
| Liu 29 | 2019 | Fair |
| Chan 10 | 2019 | Good |
| Liu 30 | 2020 | Fair |
| Wong 31 | 2020 | Fair |
| Tan 32 | 2020 | Fair |
| Li 34 | 2021 | Good |
| Liu 35 | 2021 | Good |
| Thamboo 36 | 2021 | Good |
| Lai 54 | 2021 | Fair |
| Wang 37 | 2022 | Good |
| Tsang 53 | 2022 | Good |
| Finegersh 56 | 2022 | Fair |
| Iftikhar 38 | 2023 | Poor |
| Li 33 | 2023 | Fair |
| Rampinelli 39 | 2023 | Good |
| Liu 40 | 2023 | Fair |
| Xu 41 | 2023 | Good |
| Zou 43 | 2024 | Fair |
| Wu 42 | 2024 | Fair |
| Valentini 44 | 2024 | Good |
| Chen 55 | 2024 | Fair |
| Zhao 57 | 2024 | Poor |
References
- Bray F, Ferlay J, Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. doi:https://doi.org/10.3322/caac.21492
- Badoual C. Update from the 5th edition of the World Health Organization Classification of Head and Neck Tumors: oropharynx and nasopharynx. Head Neck Pathol. 2022;16:19-30. doi:https://doi.org/10.1007/s12105-022-01449-2
- Liu J, Yu H, Sun X. Salvage endoscopic nasopharyngectomy for local recurrent or residual nasopharyngeal carcinoma: a 10-year experience. Int J Clin Oncol. 2017;22:834-842. doi:https://doi.org/10.1007/s10147-017-1143-9
- Chen Y-P, Chan A, Le Q-T. Nasopharyngeal carcinoma. Lancet. 2019;394:64-80. doi:https://doi.org/10.1016/S0140-6736(19)30956-0
- Chee J, Ting Y, Ong Y. Relapse status as a prognostic factor in patients receiving salvage surgery for recurrent or residual nasopharyngeal cancer after definitive treatment. Head Neck. 2016;38:1393-1400. doi:https://doi.org/10.1002/hed.24451
- Yang J, Song X, Sun X. Outcomes of recurrent nasopharyngeal carcinoma patients treated with endoscopic nasopharyngectomy: a meta-analysis. Int Forum Allergy Rhinol. 2020;10:1001-1011. doi:https://doi.org/10.1002/alr.22552
- Al-Sheibani S, Zanation A, Carrau R. Endoscopic endonasal transpterygoid nasopharyngectomy. Laryngoscope. 2011;121:2081-2089. doi:https://doi.org/10.1002/lary.22165
- Castelnuovo P, Nicolai P, Turri-Zanoni M. Endoscopic endonasal nasopharyngectomy in selected cancers. Otolaryngol Head Neck Surg. 2013;149:424-430. doi:https://doi.org/10.1177/0194599813493073
- Chan J, Wei W. Impact of resection margin status on outcome after salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2016;38:E594-599. doi:https://doi.org/10.1002/hed.24046
- Chan J, Wong S, Wei W. Surgical salvage of recurrent T3 nasopharyngeal carcinoma: prognostic significance of clivus, maxillary, temporal and sphenoid bone invasion. Oral Oncol. 2019;91:85-91. doi:https://doi.org/10.1016/j.oraloncology.2019.02.023
- Kuga R, Hashimoto K, Yamamoto H. Clinicopathological review of head and neck squamous cell carcinomas after neoadjuvant chemotherapy. Anticancer Res. 2024;44:4593-4603. doi:https://doi.org/10.21873/anticanres.17289
- Booth A, Clarke M, Ghersi D. An international registry of systematic-review protocols. Lancet. 2011;377:108-109. doi:https://doi.org/10.1016/S0140-6736(10)60903-8
- Page M, McKenzie J, Bossuyt P. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. doi:https://doi.org/10.1136/bmj.n71
- Hao S-P, Tsang N-M, Chang K-P. Nasopharyngectomy for recurrent nasopharyngeal carcinoma: a review of 53 patients and prognostic factors. Acta Otolaryngol. 2008;128:473-481. doi:https://doi.org/10.1080/00016480701813806
- Study quality assessment tools. Published online 2021.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. doi:https://doi.org/10.1016/0197-2456(86)90046-2
- Higgins J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. doi:https://doi.org/10.1136/bmj.327.7414.557
- Egger M, Smith G, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. doi:https://doi.org/10.1136/bmj.315.7109.629
- Chen M-Y, Wen W-P, Guo X. Endoscopic nasopharyngectomy for locally recurrent nasopharyngeal carcinoma. Laryngoscope. 2009;119:516-522. doi:https://doi.org/10.1002/lary.20133
- Rohaizam J, Subramaniam S, Vikneswaran T. Endoscopic nasopharyngectomy: the Sarawak experience. Med J Malaysia. 2009;64:213-215.
- Ko J-Y, Wang C-P, Ting L-L. Endoscopic nasopharyngectomy with potassium-titanyl-phosphate (KTP) laser for early locally recurrent nasopharyngeal carcinoma. Head Neck. 2009;31:1309-1315. doi:https://doi.org/10.1002/hed.21091
- Ho A, Kaplan M, Fee W. Targeted endoscopic salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma. Int Forum Allergy Rhinol. 2012;2:166-173. doi:https://doi.org/10.1002/alr.20111
- Chen Y-F, Wang Y-F, Wang C-P. Magnetic resonance imaging following endoscopic nasopharyngectomy with a potassium-titanyl-phosphate (KTP) laser for early locally recurrent nasopharyngeal carcinoma. Neuroradiology. 2013;55:1413-1421. doi:https://doi.org/10.1007/s00234-013-1283-1
- Emanuelli E, Albu S, Cazzador D. Endoscopic surgery for recurrent undifferentiated nasopharyngeal carcinoma. J Craniofac Surg. 2014;25:1003-1008. doi:https://doi.org/10.1097/SCS.0000000000000698
- Hsu N, Shen P, Chao S. En bloc resection concept for endoscopic endonasal nasopharyngectomy: surgical anatomy and outcome. Chin Med J (Engl). 2014;127:2934-2939.
- Weng J, Wei J, Si J. Clinical outcomes of residual or recurrent nasopharyngeal carcinoma treated with endoscopic nasopharyngectomy plus chemoradiotherapy or with chemoradiotherapy alone: a retrospective study. PeerJ. 2017;5. doi:https://doi.org/10.7717/peerj.3912
- Vlantis A, Lee D, Wong E. Endoscopic nasopharyngectomy in recurrent nasopharyngeal carcinoma: a case series, literature review, and pooled analysis. Int Forum Allergy Rhinol. 2017;7:425-432. doi:https://doi.org/10.1002/alr.21881
- Tang I, Ngui L, Ramachandran K. A 4-year review of surgical and oncological outcomes of endoscopic endonasal transpterygoid nasopharyngectomy in salvaging locally recurrent nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2019;276:2475-2482. doi:https://doi.org/10.1007/s00405-019-05522-5
- Liu Y-P, Lv X, Zou X. Minimally invasive surgery alone compared with intensity-modulated radiotherapy for primary stage I nasopharyngeal carcinoma. Cancer Commun (Lond). 2019;39. doi:https://doi.org/10.1186/s40880-019-0415-3
- Liu Q, Sun X, Li H. Types of transnasal endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma: Shanghai EENT Hospital experience. Front Oncol. 2020;10. doi:https://doi.org/10.3389/fonc.2020.555862
- Wong E, Liew Y, Loong S. Five-year survival data on the role of endoscopic endonasal nasopharyngectomy in advanced recurrent rT3 and rT4 nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol. 2020;129:287-293. doi:https://doi.org/10.1177/0003489419887410
- Tan S, Husain S, Zahedi F. Nasopharyngeal carcinoma: outcome of endoscopic nasopharyngectomy for local recurrence. UTMJ. 2020;97:14-17.
- Li W, Zhang Q, Chen F. Endoscopic surgery is superior to intensity-modulated radiotherapy in the treatment of advanced recurrent nasopharyngeal carcinoma. Int Forum Allergy Rhinol. 2023;13:140-150. doi:https://doi.org/10.1002/alr.23051
- Li W, Zhang H, Lu H. Clinical outcomes of salvage endoscopic nasopharyngectomy for patients with advanced recurrent nasopharyngeal carcinoma. Front Oncol. 2021;11. doi:https://doi.org/10.3389/fonc.2021.716729
- Liu Y-P, Wen Y-H, Tang J. Endoscopic surgery compared with intensity-modulated radiotherapy in resectable locally recurrent nasopharyngeal carcinoma: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:381-390. doi:https://doi.org/10.1016/S1470-2045(20)30673-2
- Thamboo A, Patel V, Hwang P. 5-year outcomes of salvage endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma. J Otolaryngol Head Neck Surg. 2021;50. doi:https://doi.org/10.1186/s40463-020-00482-x
- Wang Z-Q, Xie Y-L, Liu Y-P. Endoscopic nasopharyngectomy combined with internal carotid artery pretreatment for recurrent nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2022;166:490-497. doi:https://doi.org/10.1177/01945998211011076
- Iftikhar H, Awan M, Ahmed S. Endonasal endoscopic nasopharyngectomy for nasopharyngeal malignancies: a survival analysis. Egyptian J Otolaryngol. 2023;39. doi:https://doi.org/10.1186/s43163-023-00534-9
- Rampinelli V, Ferrari M, Mattavelli D. Treatment of loco-regional recurrence of nasopharyngeal carcinoma in a non-endemic area: oncologic outcomes, morbidity, and proposal of a prognostic nomogram. Front Oncol. 2023;13. doi:https://doi.org/10.3389/fonc.2023.1157584
- Liu Y, Huang N, Gao J. Endoscopic surgery versus intensity-modulated radiotherapy in locally advanced recurrent nasopharyngeal carcinoma: a multicenter, case-matched comparison. J Otolaryngol Head Neck Surg. 2023;52. doi:https://doi.org/10.1186/s40463-023-00656-3
- Xu H, Li W, Zhang H. Preliminary evidence for endoscopic surgery combined with postoperative anti-PD-1 immunotherapy in advanced recurrent nasopharyngeal carcinoma. BMC Cancer. 2023;23. doi:https://doi.org/10.1186/s12885-023-11760-y
- Wu W-B, Zhang X-B, Feng Z-K. Strategies for patients with recurrent nasopharyngeal carcinoma involved internal carotid artery who are intolerant to embolization. Rhinology. 2024;62:342-352. doi:https://doi.org/10.4193/RhinRhin23.130
- Zou X, Feng Z-K, Hua Y-J. A novel endoscopic nasopharyngectomy by low-temperature plasma radiofrequency ablation in localized recurrent nasopharyngeal carcinoma. Head Neck. 2024;46:291-299. doi:https://doi.org/10.1002/hed.27579
- Valentini M, Lambertoni A, Sileo G. Salvage endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma in a non-endemic area. Eur Arch Otorhinolaryngol. 2024;281:3601-3613. doi:https://doi.org/10.1007/s00405-024-08500-8
- King W, Ku P, Mok C. Nasopharyngectomy in the treatment of recurrent nasopharyngeal carcinoma: a twelve-year experience. Head Neck. 2000;22:215-222. doi:https://doi.org/10.1002/(sici)1097-0347(200005)22:3<215::aid-hed2>3.0.co;2-b
- Shu C, Cheng H, Lirng J. Salvage surgery for recurrent nasopharyngeal carcinoma. Laryngoscope. 2000;110:1483-1488. doi:https://doi.org/10.1097/00005537-200009000-00014
- Hao S-P, Tsang N-M, Chang C-N. Salvage surgery for recurrent nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2002;128:63-67. doi:https://doi.org/10.1001/archotol.128.1.63
- Fee W, Moir M, Choi E. Nasopharyngectomy for recurrent nasopharyngeal cancer: a 2- to 17-year follow-up. Arch Otolaryngol Head Neck Surg. 2002;128:280-284. doi:https://doi.org/10.1001/archotol.128.3.280
- Choi J, Lee W. Curative surgery for recurrent nasopharyngeal carcinoma via the infratemporal fossa approach. Arch Otolaryngol Head Neck Surg. 2005;131:213-216. doi:https://doi.org/10.1001/archotol.131.3.213
- Vlantis A, Chan H, Tong M. Surgical salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma: a multivariate analysis of prognostic factors. Head Neck. 2011;33:1126-1131. doi:https://doi.org/10.1002/hed.21585
- Chan J, Tsang R, Wei W. Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2015;37:487-492. doi:https://doi.org/10.1002/hed.23633
- Ng L, Lim C, Loh K. Long-term outcomes of nasopharyngectomy using partial maxillectomy approach. Laryngoscope. 2016;126:1103-1107. doi:https://doi.org/10.1002/lary.25777
- Tsang R, Chan W, Holsinger F. Long-term results of robotic-assisted nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2022;44:1940-1947. doi:https://doi.org/10.1002/hed.27115
- Lai Y, Li W, Zhai C. Low-grade nasopharyngeal papillary adenocarcinoma: a review of 28 patients in a single institution. Cancer Manag Res. 2021;13:1271-1278. doi:https://doi.org/10.2147/CMAR.S288007
- Chen Y, Shi Y, Yu H. Adenoid cystic carcinoma of the nasopharynx: a retrospective study of 12 cases. Ear Nose Throat J. Published online 2024. doi:https://doi.org/10.1177/01455613241259357
- Finegersh A, Said M, Deconde A. Open and endoscopic surgery improve survival for squamous and nonsquamous cell nasopharyngeal carcinomas: an NCDB cohort study. Int Forum Allergy Rhinol. 2022;12:1350-1361. doi:https://doi.org/10.1002/alr.23000
- Zhao Y, Fang J, Zhong Q. Surgical treatment and prognosis of recurrent and radiotherapy insensitive nasopharyngeal carcinoma. Braz J Otorhinolaryngol. 2024;90. doi:https://doi.org/10.1016/j.bjorl.2023.101366
- Zhang X-M, Cao J-Z, Luo J-W. Nasopharyngeal mucoepidermoid carcinoma: a review of 13 cases. Oral Oncol. 2010;46:618-621. doi:https://doi.org/10.1016/j.oraloncology.2010.06.001
- Xu T, Tang J, Gu M. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr Oncol. 2013;20:406-419. doi:https://doi.org/10.3747/co.20.1456
- Na’ara S, Amit M, Billan S. Outcome of patients undergoing salvage surgery for recurrent nasopharyngeal carcinoma: a meta-analysis. Ann Surg Oncol. 2014;21:3056-3062. doi:https://doi.org/10.1245/s10434-014-3683-9
- Bozkurt G, Turri Zanoni M, Ferrari M. Salvage surgery in nasopharyngeal cancer: unraveling the efficacy of transnasal endoscopic nasopharyngectomy for advanced stage recurrent tumors. Oral Oncol. 2024;159. doi:https://doi.org/10.1016/j.oraloncology.2024.107048
- Chen Y, Zhong N-N, Cao L-M. Surgical margins in head and neck squamous cell carcinoma: a narrative review. Int J Surg. 2024;110:3680-3700. doi:https://doi.org/10.1097/JS9.0000000000001306
- Ferrari M, Gaudioso P, Taboni S. Intraoperative surgical navigation improves margin status in advanced malignancies of the anterior craniofacial area: a prospective observational study with systematic review of the literature and meta-analysis. Eur J Surg Oncol. 2025;51. doi:https://doi.org/10.1016/j.ejso.2024.109514
- Chan R-L, Ho S-L, Chan J. Accuracy of intraoperative frozen section analysis of nasopharyngeal carcinoma resection margins: accuracy of frozen section analysis in NPC resection margins. Head Neck. 2014;36:638-642. doi:https://doi.org/10.1002/hed.23344
- Daniel R, Yan B, Chandarana S. Surgical margin definition and assessment in head and neck oncology: a cross-sectional survey of Canadian head and neck surgeons. J Otolaryngol Head Neck Surg. 2024;53. doi:https://doi.org/10.1177/19160216241296121
- Zhang L, Huang Y, Hong S. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883-1892. doi:https://doi.org/10.1016/S0140-6736(16)31388-5
- Ma S-X, Zhou T, Huang Y. The efficacy of first-line chemotherapy in recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Ann Transl Med. 2018;6. doi:https://doi.org/10.21037/atm.2018.05.14
- Wang S, Zhang Y, Yang X. Shrink pattern of breast cancer after neoadjuvant chemotherapy and its correlation with clinical pathological factors. World J Surg Onc. 2013;11. doi:https://doi.org/10.1186/1477-7819-11-166
- Ling D, Sutera P, Iarrobino N. Is multifocal regression a risk factor for ipsilateral breast tumor recurrence in the modern era after neoadjuvant chemotherapy and breast conservation therapy?. Int J Radiat Oncol Biol Phys. 2019;104:869-876. doi:https://doi.org/10.1016/j.ijrobp.2019.03.012
- Perri F, Della Vittoria Scarpati G, Caponigro F. Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 2019;12:1583-1591. doi:https://doi.org/10.2147/OTT.S188148
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 945 times
- PDF downloaded - 344 times




