Rhinology
Vol. 45: Issue 2 - April 2025
The impact of the bitter taste receptor on the predisposition to chronic rhinosinusitis
Abstract
Objectives. Genetic polymorphisms in bitter taste receptor 2 member 38 (TAS2R38), expressed in the cilia of sinonasal epithelial cells, have been proposed to be contributors to chronic rhinosinusitis (CRS).
Methods. We assessed the impact of the genetically determined TAS2R38 structure on predisposition to CRS and correlated the expression of the TAS2R38 with haplotypes. 86 patients (60 CRS patients, 26 controls) undergoing nasal surgery were enrolled. PCR to identify single nucleotide polymorphisms in genes encoding TAS2R38 were performed. TAS2R38 expression in sinus mucosa tissues was assessed by immunohistochemistry.
Results. Among CRS patients, the protective genotype PAV/PAV of the TAS2R38 was observed with the lowest frequency. Immunohistochemistry displayed significant overexpression of TAS2R38 in patients with CRS and in those with a non-functional AVI/AVI genotype. Under inflammatory conditions, TAS2R38 was found to translocate from the cell membrane.
Conclusions. Genetically determined TAS2R38 polymorphisms may influence susceptibility to CRS. The AVI haplotype seems to be an independent risk factor for CRS. Additionally, TAS2Rs and related signalling pathways might create a unique group of therapeutic targets in CRS.
Introduction
Chronic rhinosinusitis (CRS) is an extensive inflammatory process that poses substantial social and economic burden. Commonly, CRS is classified on the presence or absence of nasal polyps 1. Novel publications clearly indicate that inflammation in both CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP) is extremely heterogeneous and each phenotype can be characterised by the 3 main inflammatory endotypes (T1, T2, and T3) based on the elevation of T cell cytokines (Th1, Th2, and Th17, respectively). There has been great progress in the understanding of the pathophysiology of CRS: from the innate and adaptive immune pathways including the role of eosinophils in the course of the disease.
In addition, genetic causes may lead to the development of CRS. Patients with CRS are more likely to have a positive family history than those without CRS.
Interestingly, the bitter taste receptors, TAS2Rs, are found both in gustatory cells and in multiple areas of the body. The expression of TAS2Rs is confirmed in the oral cavity, but this is not the only location of these receptors and they are also found in respiratory system cells, testicular cells, and brain. Additionally, TAS2R has been reported in neoplastic cells. The expression of TAS2R14 and TAS2R20 has also been reported to be higher in breast cancer cells than in healthy mammary tissue 2. Carrai et al. found that cases with the AVI/AVI sequence (nonfunctional allele) were associated with an increased risk of colorectal cancer 3. Finally, it is believed that genetic polymorphisms in the bitter taste receptors (TAS2Rs or T2Rs) can contribute to CRS 4. It has been confirmed that disorders in T2R genes may contribute to increased susceptibility to upper respiratory tract infections. In particular, single nucleotide polymorphisms (SNPs) in the TAS2R38 gene may place individuals at higher risk of developing CRS.
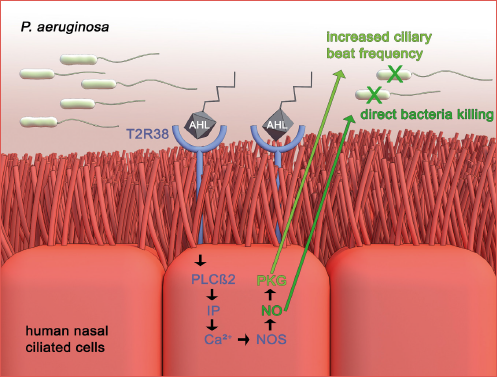
TAS2Rs are metabotropic G protein-coupled receptors (GPCR). The activity of these receptors is essential in the regulation of innate immunity. The nasal epithelium stimulation with agonists of TAS2R38 generates the calcium-dependent production of nitric oxide (NO), which plays a role as a bactericidal mediator. Another mechanism of TAS2R38 is the increase of mucociliary clearance in ciliated human sinonasal epithelial cells through the activation of protein kinase G and guanylate cyclase 5. The above mechanisms of TAS2R38 contribute to mucosal innate immunity (Cover figure).
The uniqueness of TAS2R38 is due to its genetic variants. Differences in individual sensitivity to the bitter taste of phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) depends on fluctuating receptor activity, which is associated with 3 SNPs in the TAS2R38 gene (A49P, V262A, and I296P) 2,6. The 2 more common functional alleles are characterised by the presence of proline, alanine, and valine (PAV) at positions 49, 262, and 296, and the non-functional allele with alanine, valine, and isoleucine (AVI) at the same positions. Importantly, SNPs give rise to 2 common haplotypes: (1) functional “protective” and (2) non-functional allele. Homozygotes for the taste allele sense a distinct bitter taste even at low concentrations of PTC, while homozygotes with a non-functional allele do not taste the bitterness. Recent studies revealed that heterozygous (PAV/AVI) or homozygous non-taster (AVI/AVI) genotypes have a significantly reduced response to pseudomonas quorum-sensing molecules compared with the super-taster genotype (PAV/PAV). In the PAV/PAV haplotype, there is increased production of nitrogen monoxide (NO) and ciliary beat frequency.
Previous studies have documented increased expression of TAS2R38 in tissues in patients with CRS. Our preliminary data on a small group of patients with CRS confirmed the increase in TAS2R38 in this group compared to healthy controls and showed abundant translocation of TAS2R38 into the cell nuclei in patients with CRS 7. Therefore, the aim of this study was to assess the impact of the genetically determined structure of the bitter taste receptor on the predisposition to CRS and to correlate expression and polymorphisms of the TAS2R38 gene in patients with CRS to their haplotype. Moreover, we examined the relationship between the expression of TA2R38 with bitter taste sensitivity by PROP tasting ability. Diversity in PROP tasting dysfunction might reveal clinical disparities in patients with advanced CRS.
Materials and methods
Materials
The study group consisted of 60 CRS patients undergoing functional endoscopic sinus surgery (FESS) divided into 2 subgroups: patients with (CRSwNP) and without (CRSsNP) nasal polyps. CRSwNP consisted of 25 patients aged 18 to 68 years, with a mean age of 40, and 15 women. The CRSsNP group was composed of 35 patients aged 18 to 55 years, mean age 35.2 years, and 15 women. Based on EPOS 2020 criteria 8, the presence of signs of inflammation by nasal endoscopy and the presence of clinical symptoms and radiological changes typical of CRS were required in order to be enrolled. The control group consisted of 26 patients undergoing septoplasty surgery (aged 18 to 62 years, mean age 34.1 years, 9 women) without inflammatory changes in the paranasal sinuses at CT. Fresh sinus mucosa (SM) was obtained during FESS and septoplasty from the osteomeatal complex and blood samples were collected from both groups.
Before analysis, all tissues were fixed in formalin and embedded in paraffin. The severity of disease was classified using the Lund-Mackay (L-M) scoring system graded by CT 9.
Methods
IDENTIFICATION OF SNPs IN GENES ENCODING TAS2R38 USING PCR
DNA was isolated from the peripheral blood of patients using the Qiamp© DNA Mini Kit. DNA was subjected to PCR carried out under standard conditions, in accordance with the applicable guidelines 10. The finished product, after performing the PCR reaction, was purified using ExoSap-IT© PCR Product Cleanup. This enabled the removal of smaller nucleotides and unnecessary primers. Re-amplification and purification of the final product were then carried out. The purified samples were placed in a sequencer (ABI PRISM 3130 xl Genetic Analyzer), and the finished sequences were read using the Chromas program.
IMMUNOHISTOCHEMISTRY
Samples of sinus mucosa were cut into 3.5-μm sections and then dried at 37°C for 12 hours. Deparaffinisation was performed according to a standard protocol 11. Envision Flex+, Mouse, High pH Detection System (Dako, Agilent) was used for immunohistochemistry as described by Sarnowska 12. Incubation with primary antibodies against TAS2R38 (Taste Receptor, Type 2, member 38 Antibody, Catalog No. OSR00163W, ThermoFisher Scientific) was performed for 1 hour at room temperature. Intensity and quantity of brown colour of horseradish peroxidase due to the 3,3’-diaminobenzidine (HRP-DAB) reaction determine the level of protein expression. Semi-quantitative analysis of immunohistochemistry (IHC) results was based on the calculation of the H-score (Histioscore). The H-score was calculated based on both the degree of cell staining and the percentage of stained cells (on a scale from 0 to 3, where 0 - none, 1 - weak, 2 - medium, and 3 - strong colour). The possible score ranged from 0 to 300. Formula index H-score = [1x(%cells1+) + 2x(%cells2+) + 3x(%cells3+)]
TASTE MEASURE WITH PROP SOLUTION
The study of bitter taste was carried out with a specific quantitative method based on increasing concentrations of a bitter substance. A freshly prepared solution of PROP was used in the following concentrations: 0.032, 0.1, 0.32, 1, and 3.2%. One drop of the solution was applied to the tongue, separately on the right and left sides of the tongue. The lowest concentration of the solution was recorded, at which the bitter taste was perceived correctly.
Statistical analysis
Statistical analysis was performed using the SAS 7.4 application. A p-value < 0.05 was considered significant. Correlation coefficients were calculated using the Spearman rank method. Differences between groups were evaluated using the Wilcoxon test.
Results
TAS2R38 polymorphism
We observed that all 3 genotypes of the TAS2R38 gene were present in the patient cohorts. Single peaks in the chromatograms indicated the homozygous genotypes (PAV/PAV, AVI/AVI), while double peaks indicated the heterozygous PAV/AVI genotype.
Sequences collected from the 86 patients were analysed for TAS2R38 gene polymorphisms. There were 26 control subjects in this group, 35 with CRSsNP and 25 with CRSwNP. The share of individual TAS2R38 genotypes is presented in Table I.
The most common genotype was the heterozygous PAV/AVI genotype, observed in 45% of people. In the control group, the individual genotypes had a similar frequency (31-38%). The presence of the PAV/PAV protective genotype was most frequently observed in the control group (in 31% of patients) compared to 17% of patients with CRSsNP and 24% with CRSwNP. The AVI haplotype was observed with significantly less frequently in controls (69%) than in patients with CRS (80%). Moreover, among the patients with the PAV/PAV genotype, elevated immunoglobulin E levels were found in 25% of patients and in 29% of those with the AVI/AVI genotype, and among heterozygotes an IgE score > 100 was seen in 54% of patients. No statistically significant relationship was found.
The highest average subjective severity of symptoms of CRS according to EPOS 2020 criteria was found among patients with the PAV/PAV genotype (mean 21.59). The mean severity in those with the “non-functional” AVI/AVI genotype was 34.90. A statistically significant difference between the TAS2R38 genotypes with the subjective severity of inflammatory changes according to EPOS 2020 criteria was found (p = 0.008).
When assessing the severity of inflammatory lesions on the L-M scale in sinus CT examination, it was found that the highest average intensity of inflammatory lesions was observed in patients with the heterozygous PAV/AVI genotype. PAV/PAV homozygotes had an average sinus CT score of 10.11 out of 24 possible points, while those with the AVI/AVI genotype had an average of 13.83 points. There was no statistically significant correlation between the severity of inflammatory changes on the L-M scale and genotype.
TAS2R38 expression
Tissue material collected during surgery from the osteomeatal complex was subjected to cytochemical analysis to assess the expression of TAS2R38 in the sinus mucosa. For this purpose, anti-TAS2R38 antibodies were used. The presence of TAS2R38 was indicated by brown tissue staining. IHC showed the presence of the TAS2R38 in samples from both healthy subjects and patients with CRS. Staining was much more intense in patients with CRS compared to the control group. Moreover, staining of cell nuclei was observed more often in CRS (Fig. 1).
In order to quantitatively compare the IHC results, the H-score index was calculated (Tab. II). Sequentially, the IHC analysis included the calculation of the percentage of stained cell nuclei (Tab. III).
Statistical analysis using Spearman’s rank correlation showed significantly higher expression of the TAS2R38 in patients with CRS compared to healthy subjects (R = 0.37, p = 0.015). A significant relationship was found between the percentage of stained cell nuclei of controls and CRS (R = 0.33, p = 0.034). Using the Kruskal-Wallis test, a significant relationship between the CRS and the H-score and the percentage of stained cells compared to healthy subjects was observed (Chi2 = 6.15 with p = 0.013 and Chi2 test value = 4.89 with p = 0.027, respectively).
Correlation of the TAS2R38 expression with a genotype
The results of the IHC study were analysed for the correlation of TAS2R38 expression (H-score index and percentage of cell nuclei staining) with genotype (Tabs. IV and V). In individuals with the AVI/AVI non-functional genotype, the average expression of the receptor in the examined tissues was higher than with the PAV/PAV genotype. In heterozygotes, a wide range of values were observed (Fig. 2).
PROP tasting ability in CRS patients
In our study, almost half of patients in the CRS group (48%) experienced bitter taste at only the highest concentrations (1% and 3.2% PROP), while 35% of patients sensed bitter taste at a concentration of 0.32% PROP. Bitter taste disturbances predominated in the CRSwNP group in which 63% of patients felt PROP only at concentrations of 1% and 3.2%. Moreover, none of the patients felt gustatory stimuli at the lowest level of 0.032%, and 30% of respondents sensed PROP at 0.32%. In the control group, 60% of people experienced a bitter taste even at concentrations of 0.1% and 0.32%. Only 16% of subjects felt the PROP solution only at the highest concentrations (i.e. 1% and 3.2%)
The gustometric analysis of bitter taste showed no statistically significant differences between CRS groups (Chi2 test value = 2.138, p = 0.139). However, a significant difference was obtained in the bitter taste threshold between the study groups and the control group (Chi2 value 18.780, p < 0.0001). Patients with both CRSsNP and CRSwNP had significantly higher bitter taste thresholds than healthy subjects. On the other hand, correlations were observed between the feeling of bitter taste and the genotype of patients. Mantel-Haenszel analysis for the study groups showed significant values with a value of Chi2 = 23.806 and p = 0.033. Comparison of the TAS2R38 genotype with the gustometric test results showed that PAV/PAV homozygotes (defensive-functional genotype) had a low PROP threshold. On the other hand, homozygotes with the genetically non-functional AVI/AVI genotype showed an elevated PROP threshold of 3.2%, which was present in 41% of subjects. In the gustometric test, heterozygotes obtained an even distribution of the bitter taste threshold: 51% below 1% PROP and 48% above 1% PROP (Fig. 3).
Discussion
CRS is a multifactoral disease with great social influence and different aetiological factors. The extremely high incidence of CRS is inversely proportional to the limited pharmacotherapeutic options which are based mainly on topical and general steroids, followed by FESS. Recent research confirmed the genetic background based on observations of the tendency of some patients to develop the disease. By clarifying the genetic basis of the disease, it may be possible to obtain a faster diagnosis and develop more effective treatments. Research has identified several genes underlying CRS.
One of these is the TAS2R38 gene, encoding the bitter taste receptor TAS2R38, which is also expressed in mucociliary cells of sinus mucosa. It has been demonstrated that SNPs in the TAS2R38 gene are responsible for differences in the individual susceptibility to CRS 13.
Recent publications support the hypothesis that the PAV/PAV genotype may have a protective role against gram-negative infections 14. Additionally, there is an observation concerning the higher prevalence of the AVI/AVI genotype and AVI haplotype among CRS patients compared to the healthy population of the same geographic region, suggesting that this haplotype may predispose individuals to CRS 15.
One of the first large studies was by Adappa et al. patients undergoing primary FESS. The distribution of haplotypes in the CRS on patients was 37% AVI/AVI, 54% PAV/AVI, and 8.5% PAV/PAV, which was significantly different from the distributions in the healthy control group. No important differences were found in the allelic distribution with respect to other risk factors, such as asthma or allergies 15. However, not all studies have confirmed the above hypothesis. Gallo et al. found no correlation between TAS2R38 genotypes and CRS and related comorbidities in an Italian cohort of patients with CRSsNP and CRSwNP 16. Nevertheless, a more recent report by Cantone et al. showed that the nonfunctional genotype is more frequent among CRS patients than in the general population. Their findings confirm an inverse correlation between TAS2R38 functionality and gram-negative infections in Italian patients with CRSwNP. The authors also demonstrated a relationship between in vivo microbial biofilm and TAS2R38 receptor variants 17. In Japanese patients, Takemoto et al. also found a similar distribution of polymorphisms. They also revealed revealed that TA2R38 expression in the ethmoid sinus mucosa in patients with non-eosinophilic CRS generally showed fewer T2R38-positive cells throughout the epithelial area compared to eosinophilic CRS 18. A recent publication in a Polish population by Jeruzal-Świątecka et al. confirmed that the PAV/PAV diplotypes might have some protective properties, and the AVI haplotype might predispose to the development of CRSwNP 19.
Sequencing of the gene encoding the bitter taste receptor showed that the polymorphisms described in the literature 20 were also present in our cohorts. Among all patients with CRS, 45.3% of patients had the PAV/AVI heterozygous genotype. The protective PAV/PAV was present in 23.2% of cases, and the non-protective AVI/AVI in 31.3%. These results approach the distribution obtained in classical Mendelian genetics. The presence of the PAV/PAV genotype was most frequently reported in the control group (in 31% of patients) and only in 17% of patients with CRSsNP and 24% with CRSwNP. Thus, the theory of the protective function of the PAV allele is confirmed, due to the low number of patients with CRS with the PAV/PAV genotype. Moreover, the AVI haplotype was observed significantly more often in CRS patients (80%) than in controls (69%). The authors confirmed the distribution of haplotypes in the CRS group, but there was no evidence of an increased occurrence of a given haplotype in patients with type 2 or non-type 2 inflammation.
In our study, we also found significant differences in the perception of the bitter taste of PROP between the CRS and control groups. Rowan et al. 21 indicated that the ability to sense oral bitter taste may be a simple marker of symptom severity in CRS and appears to provide individual information regarding the phenotype of patients. Moreover, Linn et al. also reported that reduced sensitivity to denatonium benzoate and PTC was observed among CRS patients with difference between CRSsNP and CRSwNP 22. Our results are similar to those reported by the above authors. In this regard, we hypothesise that taste tests may be useful as a diagnostic tool to assess the airway taste receptor function, what might indicate impaired innate immunity and predisposition to inflammatory disorders.
In addition, we also studied defense mechanisms on the haplotype of the TAS2R38 gene and the function of the receptor in rhinosinusitis. In particular, IHC analysis showed increased expression of TAS2R38 in samples from the osteomeatal complex in both CRSwNP and CRsNP. Other authors also confirmed the presence of TAS2R38 in nasal polyp tissue and the inferior turbinate nasal mucosa of CRS patients 13. However, our study is the first to confirm the significant increase of the TAS2R38 receptor in tissues from osteomeatal complex in patients with CRS.
The phenomenon of significant overexpression of TAS2R38 and its role in CRS may be explained by one of the functions of TAS2R38 causing strong intracellular NO production, which not only damages bacterial enzymes, membranes and DNA, but also increases the beat frequency of cilia. This TAS2R38 function is activated after contact with pathogens. Two major Acyl-homoserine lactones from Pseudomonas aeruginosa were confirmed to be agonists of TAS2R38. Therefore, it is most likely that TAs2R38 may function within ciliated cells in the airway as a sentinel receptor for Gram-negative bacteria, which can invasively induce a critical defensive bactericidal response. Nevertheless, the above hypotheses are only conjectures as an attempt to explain the expression of the TAS2R38 receptor, and further studies are needed.
A debatable issue requiring further research is the question of whether overexpression of TAS2R38 in CRS is the cause or the result of inflammation. In order to extend this knowledge, one of the assumptions of this study was to analyse and correlate polymorphisms of the TAS2R38 gene with expression of the bitter taste receptor in tissues to determine whether the studied phenomenon has a functional/”defensive” or non-functional role. The analysis of this aspect broadened our understanding of the role of TAS2R38 in the pathophysiology of CRS.
In the present study, the genetically determined structure of the bitter taste receptor impacted the predisposition to CRS. Patients with the non-functional genotype AVI/AVI, compared to other genotypes, had the highest average expression of the receptor in the nasal tissues. According to Piatti et al., TAS2R38 may play a role as resistant immune barrier that is active in modulating immune mechanisms, pathogen recognition and regulating the equity between commensalism and the pathogenicity of bacteria 23. Thus, we can assume that increased expression of the receptor is most likely a mechanism of local cell defense against its poor function. A greater accumulation of weak receptors is supposed to accelerate the control of inflammation. If the receptor works ineffectively, then the tissue produces more of it to achieve the effect. This phenomenon could be used for the targeted treatment of CRS in addition to biological therapy, which is increasingly used in the treatment of CRS.
Our study is the first to confirm TAR2R38 translocation from the cell membrane to cell nuclei as a result of inflammation. On IHC, staining of cell nuclei was observed more often in CRS patients than in controls, which may suggest the influence of the receptor on gene expression.
Our study is limited by the relatively small sample size. Another important limitation is that TAS2R38 gene expression was not confirmed by quantitative (RTqPCR) or semi-quantitative western blotting methods in addition to IHC analysis. Unfortunately, this was not possible due to the small amount of material collected. Nevertheless, we believe that further research on bitter taste receptors in patients with CRS population is warranted. There is great opportunity for the potential use in the diagnosis and treatment of CRS, especially in those who require more watchful care, and to improve the quality of life of patients.
Conclusions
Among patients with CRS, the protective genotype PAV/PAV is observed at the lowest frequency. The TAS2R38 receptor is overexpressed in patients with CRS, especially in its non-functional (AVI/AVI) form. The AVI haplotypes are independent risk factors for CRS. TAS2R38 translocated from the cell membrane to cell nuclei as a result of inflammation and its signaling pathways might create a unique group of therapeutic targets to treat CRS.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This study was financed by the Centre of Postgraduate Medical Education, grant number 501-1-019-56-22 to K.D. and the APC was funded by Cardinal Stefan Wyszynski University.
Author contributions
KD, KPZ: conceptualization, funding acquisition, Writing-review & editing; MS, KC: methodology: MS,KC. RJ: software; KD, KPZ, ES: formal analysis; KD, KC, ES: data curation; KPZ, MS: visualization; KD, ES: supervision; KPZ, KC: writing-original draft.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Ethics Committee, Warsaw Medical University, Poland (KB/130/2015, 2.06.2015).
Informed consent was obtained from all subjects involved in the study.
History
Received: April 1, 2024
Accepted: November 2, 2024
Figures and tables
Figure 1. Immunohistochemical staining of mucosal tissues with anti-TAS2R38 antibodies. A) CRSwNP (H-score: 85.67); B) CRSsNP (H-score: 65.68); C) control subjects (H-score 0.67); D) negative control.
Figure 2. Distribution of the Wilcoxon test for the percentage of cell staining by TAS2R38 genotype.
Figure 3. Gustometric analysis by TAS2R38 genotype.
| Genotype | Control subjects | CRSsNP | CRSwNP | Total |
|---|---|---|---|---|
| PAV/PAV | 8 (31%) | 6 (17%) | 6 (24%) | 20 (23.3%) |
| PAV/AVI | 8 (31%) | 16 (46%) | 15 (60%) | 39 (45.3%) |
| AVI/AVI | 10 (38%) | 13 (37%) | 4 (16%) | 27 (31.4%) |
| Group | H-score | SD | Median | Min | Max | Confidence interval | |
|---|---|---|---|---|---|---|---|
| -95% | +95% | ||||||
| CRSsNP | 41.1 | 35 | 34.6 | 0.3 | 131.3 | 23.1 | 59.1 |
| CRSwNP | 26.6 | 29.3 | 20.5 | 0.3 | 85.6 | 14 | 45.3 |
| Control | 9.7 | 14.8 | 3 | 0.6 | 46.9 | -1.6 | 21 |
| Group | % of cell staining | SD | Median | Min | Max | Confidence interval | |
|---|---|---|---|---|---|---|---|
| -95% | +95% | ||||||
| CRSsNP | 24.4 | 18.1 | 23.3 | 0.3 | 67 | 15.1 | 33.7 |
| CRSwNP | 17 | 14.7 | 13 | 0.3 | 42.6 | 9.1 | 24.8 |
| Control | 7.7 | 11.4 | 3 | 0.6 | 36.6 | -1 | 16.5 |
| Genotype | H-score | SD | Median | Min | Max | Confidence interval | |
|---|---|---|---|---|---|---|---|
| -95% | +95% | ||||||
| PAV/PAV | 13.7 | 18.5 | 6.6 | 0.6 | 62.6 | 1.3 | 26.2 |
| PAV/AVI | 34.9 | 35 | 26.8 | 0.3 | 131.3 | 19.4 | 50.5 |
| AVI/AVI | 37.8 | 29.4 | 41.6 | 1.6 | 88.6 | 15.2 | 60.5 |
| Genotype | % of stained cells | SD | Median | Min | Max | Confidence interval | |
|---|---|---|---|---|---|---|---|
| -95% | +95% | ||||||
| PAV/PAV | 9.3 | 10.3 | 5 | 0.6 | 33 | 2.4 | 16.3 |
| PAV/AVI | 20 | 18.2 | 16.1 | 0.3 | 67 | 11.9 | 28.1 |
References
- Klingler A, Stevens W, Tan B. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol. 2021;147:1306-1317. doi:https://doi.org/10.1016/j.jaci.2020.11.037
- Jaggupilli A, Singh N, Upadhyaya J. Analysis of the expression of human bitter taste receptors in extraoral tissues. Mol Cell Biochem. 2017;426:137-147. doi:https://doi.org/10.1007/s11010-016-2902-z
- Carrai M, Steinke V, Vodicka P. Association between TAS2R38 gene polymorphisms and colorectal cancer risk: a case-control study in two independent populations of Caucasian origin. PLoS One. 2011;6. doi:https://doi.org/10.1371/journal.pone.0020464
- Chen J, Song C, Hura N. Taste receptors in chronic rhinosinusitus, what is the evidence? A systematic review. Int Forum Allergy Rhinol. 2022;12:917-934. doi:https://doi.org/10.1002/alr.22938
- Adappa N, Howland T, Palmer J. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3:184-187. doi:https://doi.org/10.1002/alr.21140
- Kim U, Jorgenson E, Coon H. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221-1225. doi:https://doi.org/10.1126/science.1080190
- Zborowska-Piskadlo K, Stachowiak M, Rusetska N. The expression of bitter taste receptor TAS2R38 in patients with chronic rhinosinusitis. Arch Immunol Ther Exp (Warsz). 2020;68. doi:https://doi.org/10.1007/s00005-020-00593-3
- Fokkens W, Lund V, Hopkins C. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58:1-464. doi:https://doi.org/10.4193/Rhin20.600
- Fokkens W, Lund V, Mullol J. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1-12. doi:https://doi.org/10.4193/Rhino12.000
- Lorenz T. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp. Published online 2012. doi:https://doi.org/10.3791/3998
- Nirmalan N, Harnden P, Selby P. Development and validation of a novel protein extraction methodology for quantitation of protein expression in formalin-fixed paraffin-embedded tissues using western blotting. J Pathol. 2009;217:497-506. doi:https://doi.org/10.1002/path.2504
- Sarnowska E, Szymanski M, Rusetska N. Evaluation of the role of downregulation of SNF5/INI1 core subunit of SWI/SNF complex in clear cell renal cell carcinoma development. Am J Cancer Res. 2017;7:2275-2289.
- Jeruzal-Swiatecka J, Borkowska E, Laszczych M. TAS2R38 bitter taste receptor expression in chronic rhinosinusitis with nasal polyps: new data on polypoid tissue. Int J Mol Sci. 2022;23. doi:https://doi.org/10.3390/ijms23137345
- Yilmaz G, Eyigor H, Gur O. The role of TAS2R38 genotype in surgical outcomes and culturable bacteria in chronic rhinosinusitis with or without nasal polyps. Rhinology. 2023;61:54-60. doi:https://doi.org/10.4193/Rhin22.118
- Adappa N, Zhang Z, Palmer J. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3-7. doi:https://doi.org/10.1002/alr.21253
- Gallo S, Grossi S, Montrasio G. TAS2R38 taste receptor gene and chronic rhinosinusitis: new data from an Italian population. BMC Med Genet. 2016;17. doi:https://doi.org/10.1186/s12881-016-0321-3
- Cantone E, Negri R, Roscetto E. In vivo biofilm formation, gram-negative infections and TAS2R38 polymorphisms in CRSw NP patients. Laryngoscope. 2018;128:E339-E345. doi:https://doi.org/10.1002/lary.27175
- Takemoto K, Lomude L, Takeno S. Functional alteration and differential expression of the bitter taste receptor T2R38 in human paranasal sinus in patients with chronic rhinosinusitis. Int J Mol Sci. 2023;24. doi:https://doi.org/10.3390/ijms24054499
- Jeruzal-Swiatecka J, Borkowska E, Borkowska M. TAS2R38 bitter taste receptor polymorphisms in patients with chronic rhinosinusitis with nasal polyps preliminary data in Polish population. Biomedicines. 2024;12. doi:https://doi.org/10.3390/biomedicines12010168
- Dzaman K, Zagor M, Stachowiak M. The correlation of TAS2R38 gene variants with higher risk for chronic rhinosinusitis in Polish patients. Otolaryngol Pol. 2016;70:13-18. doi:https://doi.org/10.5604/00306657.1209438
- Rowan N, Soler Z, Othieno F. Impact of bitter taste receptor phenotype upon clinical presentation in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8:1013-1020. doi:https://doi.org/10.1002/alr.22138
- Lin C, Civantos A, Arnold M. Divergent bitter and sweet taste perception intensity in chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2021;11:857-865. doi:https://doi.org/10.1002/alr.22686
- Piatti G, Ambrosetti U, Alde M. Chronic rhinosinusitis: T2r38 genotyping and nasal cytology in primary ciliary dyskinesia. Laryngoscope. 2023;133:248-254. doi:https://doi.org/10.1002/lary.30112
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1400 times
- PDF downloaded - 379 times




