Reviews
Vol. 45: Issue 3 (Suppl. 1) - June 2025
Current trends in imaging for otosclerosis and the potential role of photon-counting computed tomography
Abstract
Otosclerosis is a primary otodystrophy that impacts the osseous architecture of the otic capsule within the temporal bone, resulting in progressive hearing loss. High-resolution computed tomography (HRCT) has traditionally been the gold standard imaging modality in otosclerosis, providing critical information in both diagnosis and surgical planning. However, its sensitivity varies widely. Recent advancements in imaging technology, such as ultra-high-resolution CT (UHRCT), provide higher spatial resolution and lower doses of radiation but, especially if based on cone beam CT (CBCT), face challenges in standardising bone density and are often limited by beam-hardening artefacts in the presence of metallic prostheses. Photon-counting detector CT (PCDCT) represents a promising UHRCT technology that directly converts photons into electrical signals, enhancing dose efficiency and image quality while reducing beam-hardening artefacts. Initial findings seem to indicate that PCDCT offers superior visualisation of otosclerotic foci and prosthesis positioning compared to traditional HRCT. Furthermore, PCDCT allows for less radiation exposure. This review examines the roles that HRCT and UHRCT, based both on CBCT and PCDCT, as well as magnetic resonance imaging, currently have in the imaging evaluation of otosclerosis. The findings highlight that while HRCT remains the standard, UHRCT and particularly PCDCT significantly improve the assessment capabilities, overcoming many limitations of previous technologies. Incorporating PCDCT imaging into routine clinical practice could lead to more precise diagnosis of otosclerosis, better surgical planning, and improved patient outcomes, ultimately granting more tailored and effective treatment strategies for otosclerosis, in line with the goals of precision medicine to optimise patient care.
Introduction
Otosclerosis is a primary otodystrophy that affects the osseous architecture of the otic capsule within the temporal bone 1. The primary symptom of the disease is progressive hearing loss (HL), which can be conductive or mixed and only rarely purely sensorineural 2,3. Hearing impairment is commonly bilateral, but can also occur unilaterally. Tympanometry typically reveals a good middle ear ventilation by a type A tympanogram, although stapedial reflexes are often absent or, exceptionally, exhibit a two-staged pattern (on-off stapedial reflexes). Tinnitus and vestibular symptoms may or may not accompany the HL 2. While otoscopy is usually negative, rare cases may present with Schwartze’s sign, a localised red blushing on the promontory attributed to hypervascularised otosclerotic lesions 4.
Histological manifestations of otosclerosis have been recognised for over a century. However, it is only in the last 25 years that different imaging techniques have become useful in the evaluation of this condition. Even though imaging is not imperative for diagnosis of otosclerosis 5,6, advancements in radiologic techniques have significantly increased the importance of imaging in both diagnosis and therapeutic decision-making 7-10.
In current clinical practice, high-resolution computed tomography (HRCT) represents the examination of choice for radiologic assessment of patients with otosclerosis, both in the pre- and postoperative phases (e.g., in the event of complications or when surgical revision is necessary) 7-10. Despite the several potential applications of HRCT in the evaluation of a patient with symptoms of otosclerosis, specific criteria for its use have not been clearly defined in the literature 11. Moreover, other imaging modalities, such as magnetic resonance imaging (MRI) 12,13 or even densitometry 14,15, can play a role in assessing specific aspects of this disease.
This review examines the current state of the art in imaging for otosclerosis, discussing the importance for clinical applications. Furthermore, it focuses on the impact of new technological improvements on diagnosing, planning treatment, and enhancing patient outcomes, also reporting on experience with the novel photon counting detector CT (PCDCT) technology in otosclerosis evaluation.
High-resolution computed tomography
HRCT involves acquiring images (thickness mostly 0.6 mm) with a high spatial frequency reconstruction algorithm to evaluate and characterise various conditions affecting different organs, particularly the petrous bone. HRCT is commonly conducted using multidetector CT (MDCT) scanners, which can acquire near-isotropic data.
HRCT is currently the most used imaging technique for pre- and postoperative assessment of otosclerosis and is widely considered the gold standard in imaging evaluation of this condition 5,16-20. It provides clinicians with crucial information for making therapeutic decisions, especially in atypical or ambiguous clinical presentations, and plays a pivotal role in enabling differential diagnosis.
Radiologically, otosclerosis is categorised into 2 types: fenestral and retro-fenestral. Generally, cochlear forms are preceded by the fenestral form, leading some authors to consider them as a pathologic continuum 5,21. Fenestral otosclerosis accounts for 70-80% of cases, primarily presenting as pure form rather than the rarer retro-fenestral form 1,22,23.
Through cross-sectional X-rays, HRCT can effectively highlight both the otospongiotic and sclerotic phases of otosclerosis. Otospongiotic foci appears as hypodense regions with decalcification within the otic capsule 22,24,25. False negatives can be correlated with small lesions or those in sclerotic stages characterised by attenuation similar to adjacent normal bone 7.
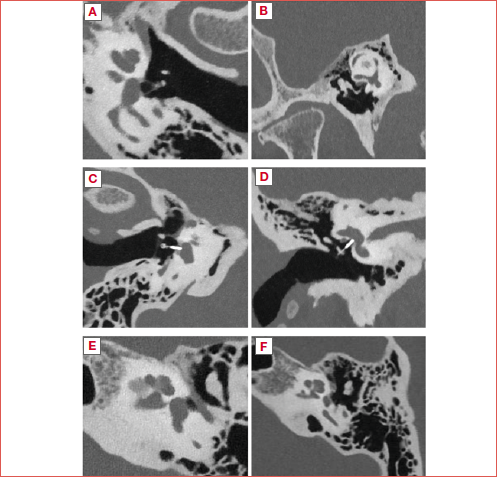
Specifically, in the fenestral form of otosclerosis, bone remodelling foci typically localise along the medial tympanic cavity wall, predominantly at the level of the fissula ante fenestram, and occasionally extending to the promontory or round window (Fig. 1A). Less frequently, foci may be observed in the tympanic segment of the facial bony canal 10. Advanced fenestral otosclerosis may exhibit complete thickening of the entire platina. Retro-fenestral otosclerosis, on HRCT, often shows variably dense areas throughout the petrous bone. Notably, the cochlea may display a characteristic double-ring appearance known as the Valvassori’s sign (Fig. 1B), and additional involvement of other labyrinthine structures, the facial canal, or the internal auditory canal are commonly observed.
One of the characteristic lesions associated with otosclerosis involving the internal auditory canal (IAC) is the diverticulus, which manifests as an erosion in the anterior canal wall and is considered a form of “cavitary otosclerosis” 26-28. It is thought to be present in the 70% of the otosclerotic petrous bone with IAC involvement 29. As well as with HRCT, its presence has been described in post-mortem otopathological studies 29. However, IAC diverticula have been documented in patients lacking radiographic evidence of fenestral or retro-fenestral otosclerosis and even in histologically normal temporal bones from individuals with normal hearing 26,29 (Fig. 1C).
A study conducted by Wells and colleagues 26 on 97 petrous bones studied with both HRCT and histopathologic section demonstrated that, while not pathognomonic, IAC diverticula are frequently observed in cases of otosclerosis, particularly when they are sizable. Similar results were reported in the study by Wang and coworkers 30. A study by Pippin and colleagues 31 examining 807 petrous bone HRCT scans revealed that the presence of diverticula correlated with HL, regardless of whether otosclerosis signs were present. Intriguingly, sensorineural HL (SNHL) showed a higher association with IAC diverticulum compared to traditional conductive or mixed findings of otosclerosis, both in petrous bones with or without other radiological signs of otosclerosis. This association was not confirmed by the latter study of Burd and colleagues 32, who concluded that there are no clear audiometric implications in the presence and even the morphology of IAC diverticula.
According to the study by Yagi and colleagues 18, otosclerotic foci are distributed in the anterior portion of the oval window (essentially at the level of the fissula ante fenestram) in more than 95% of cases in their cohort, in the anterior segment of the IAC (often defining the diverticulus) in 46.8%, and in the pericochlear area in 26%.
Another aspect to be evaluated with HRCT is the thickness of the otic capsule contour and the anterolateral to the anterior margin of the oval window. Sanghan and colleagues 33 hypothesised and demonstrated that individuals with otosclerosis exhibit a measurable increase in otic capsule thickness adjacent to the anterolateral aspect of the anterior margin of the oval window on CT compared to individuals with normal hearing.
The link between radiologic findings and audiometric results in otosclerosis has been explored by many authors, although there is still not a clear consensus regarding this relationship. Mangia and coworkers 34, evaluating the imaging and audiometric findings of 40 ears with surgically confirmed otosclerosis, found that ears with endosteal extension of the foci or with multiple affected sites within the otic capsule had worse bone-conduction hearing thresholds, and that mixed fenestral and retro-fenestral disease was associated with higher pure tone audiometric values. On the other hand, Zanini et al. 35 found no significant correlation between the location of otospongiotic foci and air conduction, bone conduction, or air-bone gap, disconfirming the thesis by Mangia et al. 34. Intriguingly, the otospongiotic involvement of the round window, especially if it is fully obliterated, is reported to be associated with worse bone thresholds both in the pre- and postoperative periods 36,37. Various grading systems for otosclerosis have been developed based on HRCT findings, but none have gained wide acceptance. For example, Rotteveel et al. 38 introduced a system classifying otosclerosis into fenestral and retro-fenestral subtypes.
However, the sensitivity of HRCT in detecting otosclerosis varies across studies. In a systematic review on HRCT sensitivity conducted by Wegner and colleagues 6, based on 7 studies of moderate to high relevance and moderate to low risk of bias, reported sensitivities ranged from 60% to 95%, with high specificity that was 100% in two studies. Moreover, these authors demonstrated that the positive predictive value (PPV) of HRCT was particularly high in cohorts with a high prevalence of the disease, and that diagnostic performance was better in more recent studies, possibly due to technological advancements. Similarly, in another systematic review, Kanzara and Virk 19 reported that HRCT had a sensitivity of 58%, and was also associated with high specificity (95%) and PPV (92%).
The wide variation of the sensitivity of HRCT reported in the literature may be related to differences in image quality, slice thickness, and study protocols, as well as radiologist experience in petrous bone imaging 39,40. From these findings, it clearly emerges that adequate study quality and protocol, as well as dedicated radiologists, should be advised 17.
Preoperative HRCT evaluation
In preoperative evaluation with HRCT for stapedial surgery, several factors need to be considered. Firstly, the size of the oval window niche, potentially reduced by osseous hypertrophy, congenital anomalies, or otosclerotic foci, requires precise assessment since it can impact postoperative results. Ukkola-Pons et al. 41 established a minimal normative value of 1.4 mm for the niche height, below which the risk of surgical difficulties increases. Additionally, the potential obliteration of the round window by otosclerotic foci demands careful consideration as it can negatively affect post-surgical outcomes 21,24,42,43. Extensive damage to the otic capsule and inner hearing canal must also be evaluated 42.
The relationship between the facial nerve and the oval window, particularly in cases of prolapse or dehiscence, necessitates careful assessment due to its significant impact on surgical feasibility, as does the presence of a very high jugular bulb. Integrating preoperative assessments with HRCT into the clinical workflow can enhance the surgeon’s ability to anticipate and assess potential intraoperative challenges and estimate outcomes more accurately 7.
Another relevant aspect is the concomitant presence of otosclerosis and superior canal dehiscence. Superior canal dehiscence can present with variable symptoms including vertigo triggered by sound pressure or head movement, autophony, conductive hyperacusis, pulsatile tinnitus, and conductive HL 44. Given the importance of appropriate differential diagnosis between the 2 conditions joined by the presence of conductive HL, some authors have reported that outcomes of stapes surgery in patients with concurrent superior canal dehiscence syndrome and otosclerosis are not straightforward concerning the probability of successful surgery in this particular group of patients 45,46. A recent systematic review concluded that although the length and location of the dehiscence might inform surgical decisions, it is not possible to draw definitive conclusions about the appropriate indications for surgical treatment due to the limited number of cases with adequate data reported in the literature 47.
Preoperative HRCT assessment can also rule out conditions that can mimic otosclerosis, such as specific inner ear malformations (IEM) or ossicular chain issues. Incomplete partition types II and III, as well as some forms of cochlear hypoplasia (mostly types II, III, and IV), can be associated with mixed or pure conductive HL, type A tympanogram, and lack of stapedial reflexes due to increased endolymph pressure or congenital alterations of articulation between the stapes and oval window 48. An interruption of the ossicular chain occurring laterally from the stapedial muscle insertion can also be associated with conductive HL, normal tympanogram, and absent stapedial reflexes.
Postoperative HRCT assessment of otosclerosis
In cases of suboptimal outcomes following stapes surgery or when a patient experiences hearing deterioration over time, an imaging study is advisable. In such cases, HRCT can evaluate the placement of the stapedial prosthesis and any potential dislocation. Additionally, HRCT can assess other possible issues such as erosion of the long process of the incus – a frequent cause of stapedial prosthesis dislocation – or the presence of granulation tissue, a perilymphatic fistula, or a pneumolabyrinth (Fig. 1D).
If preoperative HRCT was not conducted, imaging can also reveal unexpected conditions that may have limited the surgical outcome, such as round window obliteration 49 or a concomitant and previously undiagnosed superior semicircular canal dehiscence 45,50. Rarely, in cases of long-term hearing deterioration, HRCT can reveal progression of the disease with a complete subversion of the otic capsule (Fig. 1E) 51.
HRCT has also been used to check the postoperative positioning of stapes prostheses and correlate intravestibular protrusion with surgical outcomes. The criteria for measuring intravestibular protrusion of the prosthesis vary among different studies, as does the measure of insertion considered normal. Williams and Ayache 52 set the limit at 1 mm, Rangheard et al. 53 at 2 mm, and Whetstone et al. 49 at 50% of the vestibule width. Meanwhile, the depth of non-metallic prosthesis tips in the vestibule is thought to be generally underestimated 54, and depth insertion of metal prostheses tends to be overestimated due to beam hardening artifacts caused by the metal 54-56. Because of the inhomogeneous criteria, as well as the technical difficulties due to low spatial resolution and the predisposition to artefacts, in general, HRCT is yet not considered as a reliable method for the evaluation of intravestibular protrusion of stapes prostheses 55,56.
Is pre- or postoperative imaging assessment necessary?
Given the frequency of a clear clinical appearance of otosclerosis, the use of preoperative assessment with CT can be questioned. As previously discussed, the sensitivity of HRCT can be suboptimal, and it is economically and dosimetrically costly. However, pre- and postoperative CT highlight information for surgical planning and identify concomitant or concurrent conditions that may limit or have limited surgical outcomes, providing the surgeon with greater knowledge of potential surgical difficulties or suboptimal outcomes and giving the patient the best chance of an uneventful surgery. This aspect justifies the suggestion of implementing HRCT whenever otosclerosis is associated with atypical symptoms such as autophony, imbalance, vertigo, oscillopsia, hyperacusis, and aural fullness 11.
From a national survey carried out in the US in 2022, 35.3% of surgeons reported routine use of CT, with a significant difference between academic and private practice respondents, especially focusing on patients with vestibular complaints, childhood HL, possible advanced otosclerosis, or any atypical symptoms 57.
In his systematic review, Wegner 6 reported that CT might not be essential to confirm otosclerosis in patients with a high clinical suspicion based on conductive HL. Instead, its utility might be better realised in evaluating the extent of the disease or planning surgical interventions rather than serving as a primary diagnostic tool.
In our opinion, a proactive strategy utilising an imaging evaluation, especially in patients with atypical presentations of otosclerosis, can enhance precision in counselling, and in anticipating surgical outcomes and functional results. By adopting a comprehensive preoperative evaluation, even with the best achievable imaging, it is more likely to achieve improved surgical efficiency and decrease intraoperative risks.
Ultra-high-resolution CT
The advent of ultra-high-resolution CT (UHRCT) represents a significant step forward in imaging technologies. This advancement is primarily driven by the introduction of more sophisticated detector systems, such as enhanced ultra-small multi-slice detectors, flat-panel detectors, or the new photon-counting technology detectors, which allow for the capture of images at finer slice thicknesses compared to traditional HRCT. These advanced detectors ensure a higher matrix size during image reconstruction, resulting in clearer and more detailed images, and facilitating better visualisation of minute anatomical structures and pathologies.
Given that the slice thickness of conventional HRCT is typically 0.6 mm, this level of spatial resolution may be inadequate for accurately delineating otosclerotic foci smaller than 1 mm 8,40. This limitation underlines the need for more advanced imaging techniques that are capable of capturing finer details. In this context, Akazawa and colleagues 58 analysed the performance of UHRCT based on ultra-small multi-slice detectors. They measured the thickness of the stapes footplate in patients with otosclerosis finding a significant increase in thickness in otosclerosis patients compared to control subjects (0.60 ± 0.09 mm vs 0.46 ± 0.04 mm; p < .001). Furthermore, the thickness at the midpoint, where the interobserver variability was lowest, correlated well with surgical difficulty during stapedotomy.
UHRCT with cone beam CT technology
Cone beam CT (CBCT) has become increasingly popular for temporal bone imaging due to its capacity to generate high-spatial-resolution images with low radiation doses 59,60. It is mainly based on the emission of a cone-shaped radiant beam (not fan-shaped as in conventional CT) and a solid detector that can be shaped into a large surface panel (flat panel).
The slice thickness of CBCT can range from 0.075 to 0.5 mm 61. However, a significant challenge in using CBCT for bone density assessment is the technical variability in pixel values. Compared to standard multidetector CT, CBCT is more prone to produce regional artefacts. These artefacts are primarily due to off-axis X-ray beam projections, beam hardening, and scatter radiation. They are especially problematic when high-density materials are present within the beam path but outside the reconstructed field of view, producing very intrusive beam-hardening artefacts 62, such as in postoperative metallic stapedial prosthesis positioning evaluation. In their prospective study, Redfors et al. 63 compared the diagnostic capabilities of CBCT and HRCT in patients with otosclerosis who underwent stapedectomy 30 years prior. The authors found that CBCT is an effective and reliable imaging technique to detect hypodense otosclerotic lesions in the otic capsule, and was comparable to HRCT. On the other hand, Liktor et al. 64 reported that while CBCT exhibits high sensitivity for histologically confirmed active fenestral otosclerosis, it is significantly less effective at detecting retro-fenestral lesions. In another study by the same group, CBCT was found to be effective in detecting active otosclerosis with a sensitivity of 100%, but its sensitivity dropped to 0% for inactive otosclerosis. Furthermore, CBCT did not have the ability to detect retro-fenestral lesions, marking a significant limitation compared to HRCT. The general sensitivity of CBCT was 61.3% for all cases of otosclerosis, which was less than the sensitivity shown by traditional HRCT 65. Only recently, in their retrospective case-control study, Deng and colleagues 66, calibrating CBCT pixel values using 3 internal references to obtain a relative attenuation ratio that allows quantitative assessment of bone density, reported a high sensitivity (97.3%) and specificity (97.1%) of CBCT to diagnose otosclerosis. Another recent study by Xu and colleagues 67 evaluated the diagnostic capabilities of cone beam based UHRCT and standard HRCT in detecting isolated fenestral otosclerosis. UHRCT showed a sensitivity of 100% when images were evaluated by dedicated neuroradiologists and 87.5% if evaluated by general radiologists, while traditional HRCT demonstrated lower sensitivities, which was 89.2% for neuroradiologists and 41.5% for general radiologists. Significant differences in sensitivity between UHRCT and HRCT were reported, with UHRCT proving more effective in detecting smaller foci under 1 mm. However, the limitations of CBCT in standardising bone density and the subsequent difficulties in evaluating retro-fenestral otosclerotic lesions, as well as the noticeable impact in evaluating prosthesis positioning given by beam hardening artefacts, make this UHRCT study suboptimal for otosclerosis imaging evaluation.
UHRCT with photon-counting detector CT technology
On the other hand, photon-counting detector CT (PCDCT) is an emerging technology with promising results in improving clinical imaging 68,69. It directly converts photons into an electric signal, thereby recording each individual photon, allowing for more dose-efficient and high-spatial-resolution imaging 70,71. Additionally, PCDCT assigns uniform weighting to each detected photon regardless of its energy level, enhancing the signal-to-noise ratio and reducing beam-hardening artefacts, thereby improving overall image quality 72,73. Furthermore, Zhou et al. 74 demonstrated in a previous cadaver study that PCDCT allows for approximately a 50% dose reduction in radiation.
In a further study by Benson and colleagues 75, 13 patients underwent temporal bone imaging with both traditional HRCT and UHRCT with PCDCT. Their findings clearly reported that PCDCT provides higher resolution images and requires lower radiation doses compared to traditional HRCT.
As far as we know, no studies on the performance of PCDCT in otosclerosis evaluation have yet been published. Herein, we report a recent experience with PCDCT in the pre- and postoperative evaluation of 6 patients with otosclerosis using a dual-source CT scanner Photon Counting Naetom Alpha (Siemens-Healthineers): 140 kV, mean volume CT dose index = 27.17 [SD, 1.4] mGy, pitch = 0.55, rotation time = 1 second using the high-resolution mode (120 x 0.2 mm collimation) with a dedicated sharp Hr 92 kernel and the smallest section thickness of impressive 0.2 mm.
The Cover figure illustrates the high quality of image acquisition, displaying a detailed representation of the foci as well as all the anatomical structures critical for surgical planning, addressing the limitations of UHRCT based on CBCT reported earlier. However, our current sample size is too limited to reliably assess the specificity and sensitivity of this method in evaluating otosclerosis.
The main advantages of executing an UHRCT with PCDCT are:
- the ease of detection and characterisation of foci’s extension due to the very high spatial resolution and standardised density value;
- the precision in the evaluation of stapedial prosthesis positioning due to the absolute reduction of beam hardening artefacts;
- a reduction of the dose vs. traditional HRCT.
Some of the most interesting findings to be anecdotally reported were:
- a typical extension of the classical fissular otospongiotic area in a caudal and rostral direction with involvement of the lateral wall of the vestibule;
- an impressive rate of association between superior semicircular canal dehiscence in patients with otosclerosis, mainly from a dilatation of the superior petrosal sinus, accounting for one third of the evaluated subjects (Fig. 1F);
- the frequent presence of an otospongiotic area in the lateral portion of the anterior wall of the IAC that often hints at a more or less pronounced diverticulum. This finding could support the hypothesis that such a finding is nothing more than a very punctual area of cavitary otosclerosis derived from the evolution of an otospongiotic focus.
It is the authors’ opinion that such a promising technique could be considered a game-changer in pre- and postoperative imaging evaluation of otosclerotic patients and could also contribute to a better understanding of the aetiopathologic evolution of otosclerosis.
Magnetic resonance imaging
Although MRI is not the primary imaging modality for evaluating otosclerosis, it can reveal specific changes such as a pericochlear halo of intermediate signal intensity on T1-weighted sequences with pericochlear enhancement in post-contrast sequences 12,13,21,43. This enhancement is thought to occur due to the passage of contrast into numerous vessels within the otosclerotic foci 25. Occasionally, T2 hyperintensity can also be observed 76.
Purohit and coworkers 77 explored the use of MRI as a diagnostic tool for otosclerosis. The study was retrospective, analysing 13 cases from KU Leuven University Hospitals where MRI was used as the primary diagnostic tool instead of HRCT. The findings indicated that MRI could reveal subtle signs of otosclerosis, particularly when specific imaging features like intermediate T1 signal and post-contrast enhancement in perilabyrinthine or pericochlear regions are present. The study concluded that although MRI should not be the first choice for diagnosing otosclerosis, it can effectively detect the condition in patients where otosclerosis was not initially considered. Thus, MRI can lead to more targeted use of HRCT and help confirm the diagnosis.
Moreover, as our group has previously reported 12,13 the use of MRI 3D-FLAIR sequences before and after gadolinium administration can effectively evaluate cochlear damage caused by the disease. The presence of endocochlear hyperintensity and post-contrast enhancement are considered good predictors of permeability changes in the blood-labyrinth barrier. These labyrinthine disturbances might correlate with clinical parameters such as disease duration and the degree of cochlear damage as measured by audiometric thresholds. Our findings represent a significant advancement in understanding the pathogenic mechanisms underlying SNHL in otosclerosis. Specifically, the hyperintensity observed on 3D-FLAIR sequences likely reflects areas of active otospongiotic lesions where increased vascular permeability allows gadolinium to penetrate the cochlear tissues. This hyperintensity can be viewed as a biomarker of active disease, indicating ongoing inflammation and remodelling processes within the cochlea. The correlation between these MRI findings and clinical parameters such as duration of disease and audiometric thresholds supports the hypothesis that these permeability changes contribute directly to the sensorineural component of HL observed in otosclerosis patients.
Our research also provided a possible in vivo explanation for the pathogenesis of SNHL in otosclerosis by demonstrating that these imaging findings are not just incidental but are significantly associated with clinical deterioration. By quantifying the extent of cochlear damage through imaging, we can better predict the progression of HL and potentially identify patients who might benefit from early therapeutic interventions aimed at stabilising or reversing these permeability changes. This understanding could lead to more targeted treatment strategies, improving outcomes for patients with otosclerosis.
Additionally, Vicente and colleagues 78 have explored the use of MRI to monitor clinical treatment responses in otosclerosis. Employing a standard MRI protocol without 3D-FLAIR images, they observed a decrease in enhancement within active bone otospongiotic lesions on T1-weighted post-Gd sequences following drug treatment with sodium alendronate or sodium fluoride. It is proposed that incorporating a 3D-FLAIR sequence with or without gadolinium could be a valuable method to specifically identify active endocochlear damage in patients with otosclerosis, potentially enhancing the effectiveness of drug therapy. Although the efficacy of drug therapy in treating retro-fenestral otosclerosis has not been conclusively demonstrated, this imaging protocol could offer a new endpoint for evaluating the impact of treatment. Finally, when considering cochlear implantation, MRI is helpful for assessing cochlear patency, providing essential insights for surgical planning 12.
Conclusions
HRCT represents the gold standard for imaging evaluation of otosclerosis. Historically, however, it was considered nonessential due to the typically clear clinical presentation and well-defined audiological diagnostic features of otosclerosis, which could often be confirmed intraoperatively. Furthermore, systematic reviews have highlighted the suboptimal performance of HRCT, primarily due to technical limitations that resulted in limited spatial definition and consequently low sensitivity.
On the other hand, UHRCT and especially PCDCT appear to overcome the limitations of previous imaging modalities in the evaluation of otosclerotic patients. With their extraordinary spatial definition and minimal beam-hardening artefacts in the presence of metallic stapedial prostheses, PCDCT holds great promise to become the imaging modality of choice for otosclerosis both pre- and postoperatively.
The advent of PCDCT may lead clinicians to re-evaluate the role of imaging in the clinical management of otosclerosis, especially in the context of precision medicine. Enhanced imaging capabilities may facilitate more accurate diagnosis, better surgical planning, and improved postoperative assessment, ultimately leading to more tailored and effective treatment strategies for patients with otosclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
FL: conceptualization, methodology, formal analysis, data curation, writing – original draft preparation; FF, LB, SB: validation; OM: investigation; FL, SDC, FC: data curation; SC: writing – review and editing; SC, SB: visualization; all the authors: supervision and project administration.
All authors have read and agreed to the published version of the manuscript.
Ethical consideration
This study was approved by the Institutional Ethics Committee. Local Review Board do not release acceptance codes for retrospective studies based on clinical practice measures. The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: September 30, 2024
Accepted: April 7, 2025
Figures and tables
Figure 1. Computed tomography pictures in otosclerosis. A) Otosclerotic focus localised at the level of fissula ante fenestram of a right ear; B) Valvassori’s sign: double ring appearance of otic capsule in a retrofenestral form of otosclerosis of a left ear; C) Internal auditory canal diverticulus on a right ear; D) Right ear pneumolabyrinth; E) A complete subversion of the otic capsule in an advanced otosclerosis in a left ear; F) Superior semicircular canal dehiscence from the dilatation of the superior petrosal sinus in a left ear.
References
- Vicente A de O, Yamashita H, Albernaz P. Computed tomography in the diagnosis of otosclerosis. Otolaryngol Head Neck Surg. 2006;134:685-692. doi:https://doi.org/10.1016/j.otohns.2005.11.030
- Foster M, Backous D. Clinical evaluation of the patient with otosclerosis. Otolaryngol Clin North Am. 2018;51:319-326. doi:https://doi.org/10.1016/j.otc.2017/11.004
- Browning G, Gatehouse S. Sensorineural hearing loss in stapedial otosclerosis. Ann Otol Rhinol Laryngol. 1984;93:13-16. doi:https://doi.org/10.1177/000348948409300104
- Peng K, House J. Schwartze sign. Ear Nose Throat J. 2018;97. doi:https://doi.org/10.1177/014556131809700315
- Lee T, Aviv R, Chen J. CT grading of otosclerosis. AJNR Am J Neuroradiol. 2009;30:1435-1439. doi:https://doi.org/10.3174/ajnr.A1558
- Wegner I, van Waes A, Bittermann A. A systematic review of the diagnostic value of CT imaging in diagnosing otosclerosis. Otol Neurotol. 2016;37:9-15. doi:https://doi.org/10.1097/MAO.0000000000000924
- Gredilla Molinero J, Mancheño Losa M, Santamaría Guinea N. Update on the imaging diagnosis of otosclerosis. Radiologia. 2016;58:246-256. doi:https://doi.org/10.1016/j.rx.2016.04.008
- Mangia L, Coelho LO de M, Carvalho B. Imaging studies in otosclerosis: an up-to-date comprehensive review. Int Arch Otorhinolaryngol. 2021;25:E318-E327. doi:https://doi.org/10.1055/s-0040-1715149
- Wolfovitz A, Luntz M. Impact of imaging in management of otosclerosis. Otolaryngol Clin North Am. 2018;51:343-355. doi:https://doi.org/10.1016/j.otc.2017.11.005
- Kösling S, Plontke S, Bartel S. Imaging of otosclerosis. Rofo. 2020;192:745-753. doi:https://doi.org/10.1055/a-1131-7980
- Varadarajan V, Antonelli P. Is preoperative computed tomography necessary or useful for primary stapes surgery?. Laryngoscope. 2021;131:703-704. doi:https://doi.org/10.1002/lary.28732
- Berrettini S, Lombardo F, Bruschini L. 3D fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging at different stages of otosclerosis. Eur Arch Otorhinolaryngol. 2018;275:2643-2652. doi:https://doi.org/10.1007/s00405-018-5093-2
- Lombardo F, De Cori S, Aghakhanyan G. 3D-Flair sequence at 3T in cochlear otosclerosis. Eur Radiol. 2016;26:3744-3751. doi:https://doi.org/10.1007/s00330-015-4170-9
- Mangia L, Lucca de Oliveira S, Carvalho B. Measuring the density of the fissula antefenestram and the section of the basal turn of the cochlea: are they useful in the radiological diagnosis of otosclerosis?. J Otol. 2022;17:84-89. doi:https://doi.org/10.1016/j.joto.2022.01.001
- Fang Y, Chen W, Ren L-J. Stability of computed tomography densitometry in patients with otosclerosis: a two-year follow-up. J Otol. 2022;17:39-45. doi:https://doi.org/10.1016/j.joto.2021.10.003
- Quesnel A, Moonis G, Appel J. Correlation of computed tomography with histopathology in otosclerosis. Otol Neurotol. 2013;34:22-28. doi:https://doi.org/10.1097/MAO.0b013e318277a1f7
- Bassiouni M, Bauknecht H-C, Muench G. Missed radiological diagnosis of otosclerosis in high-resolution computed tomography of the temporal bone – Retrospective analysis of imaging, radiological reports, and request forms. JCM. 2023;12. doi:https://doi.org/10.3390/jcm12020630
- Yagi C, Morita Y, Takahashi K. Otosclerosis: anatomical distribution of otosclerotic loci analyzed by high-resolution computed tomography. Eur Arch Otorhinolaryngol. 2019;276:1335-1340. doi:https://doi.org/10.1007/s00405-019-05385-w
- Kanzara T, Virk J. Diagnostic performance of high resolution computed tomography in otosclerosis. WJCC. 2017;5:286-291. doi:https://doi.org/10.12998/wjcc.v5.i7.286
- Dudau C, Salim F, Jiang D. Diagnostic efficacy and therapeutic impact of computed tomography in the evaluation of clinically suspected otosclerosis. Eur Radiol. 2017;27:1195-1201. doi:https://doi.org/10.1007/s00330-016-4446-8
- Swartz J. The otodystrophies: diagnosis and differential diagnosis. Semin Ultrasound CT MR. 2004;25:305-318. doi:https://doi.org/10.1053/j.sult.2004.04.001
- Shin Y, Fraysse B, Deguine O. Sensorineural hearing loss and otosclerosis: a clinical and radiologic survey of 437 cases. Acta Otolaryngol. 2001;121:200-204. doi:https://doi.org/10.1080/000164801300043505
- Veillon F, Riehm S, Emachescu B. Imaging of the windows of the temporal bone. Semin Ultrasound CT MR. 2001;22:271-280. doi:https://doi.org/10.1016/s0887-2171(01)90011-3
- Lagleyre S, Sorrentino T, Calmels M-N. Reliability of high-resolution CT scan in diagnosis of otosclerosis. Otol Neurotol. 2009;30:1152-1159. doi:https://doi.org/10.1097/MAO.0b013e3181c2a084
- Valvassori G. Imaging of otosclerosis. Otolaryngol Clin North Am. 1993;26:359-371.
- Wells D, Knoll R, Kozin E. Otopathologic and computed tomography correlation of internal auditory canal diverticula in otosclerosis. Otol Neurotol. 2022;43:E957-E962. doi:https://doi.org/10.1097/MAO.0000000000003665
- Makarem A, Linthicum F. Cavitating otosclerosis. Otol Neurotol. 2008;29:730-731. doi:https://doi.org/10.1097/MAO.0b013e3181799763
- Makarem A, Hoang T-A, Lo W. Cavitating otosclerosis: clinical, radiologic, and histopathologic correlations. Otol Neurotol. 2010;31:381-384. doi:https://doi.org/10.1097/MAO.0b013e3181d275e8
- Muelleman T, Maxwell A, Lopez I. Histopathologic characteristics of internal auditory canal diverticula. Otol Neurotol. 2019;40:E653-E656. doi:https://doi.org/10.1097/MAO.0000000000002256
- Wang F, Yoshida T, Shimono M. Significance of internal auditory canal diverticula in ears with otosclerosis. Acta Otolaryngol. 2018;138:1066-1069. doi:https://doi.org/10.1080/00016489.2018.1521526
- Pippin K, Muelleman T, Hill J. Prevalence of internal auditory canal diverticulum and its association with hearing loss and otosclerosis. AJNR Am J Neuroradiol. 2017;38:2167-2171. doi:https://doi.org/10.3174/ajnr.A5399
- Burd C, Pai I, Pinto M. Morphological comparison of internal auditory canal diverticula in the presence and absence of otospongiosis on computed tomography and their impact on patterns of hearing loss. Neuroradiology. 2021;63:431-437. doi:https://doi.org/10.1007/s00234-020-02606-6
- Sanghan N, Chansakul T, Kozin E. Retrospective review of otic capsule contour and thickness in patients with otosclerosis and individuals with normal hearing on CT. AJNR Am J Neuroradiol. 2018;39:2350-2355. doi:https://doi.org/10.3174/ajnr.A5892
- Mangia L, Salvador G, Amadeu N. Radiological parameters and audiometric findings in otosclerosis: is there any relationship?. J Laryngol Otol. 2023;137:68-75. doi:https://doi.org/10.1017/S0022215121003947
- Zanini O, Coelho L, Hamerschmidt R. High-resolution computed tomography and pure-tone audiometry in patients with otosclerosis in the spongiotic phase. J Laryngol Otol. 2023;137:490-495. doi:https://doi.org/10.1017/S0022215122001608
- Karosi T, Csomor P, Sziklai I. The value of HRCT in stapes fixations corresponding to hearing thresholds and histologic findings. Otol Neurotol. 2012;33:1300-1307. doi:https://doi.org/10.1097/MAO.0b013e31826352ad
- Shin Y, Deguine O, Cognard C. Reliability of CT scan in the diagnosis of conductive hearing loss with normal tympanic membrane. Rev Laryngol Otol Rhinol (Bord). 2001;122:81-84.
- Rotteveel L, Proops D, Ramsden R. Cochlear implantation in 53 patients with otosclerosis: demographics, computed tomographic scanning, surgery, and complications. Otol Neurotol. 2004;25:943-952. doi:https://doi.org/10.1097/00129492-200411000-00014
- Yamashita K, Hiwatashi A, Togao O. Additive value of ‘otosclerosis-weighted’ images for the CT diagnosis of fenestral otosclerosis. Acta Radiol. 2017;58:1215-1221. doi:https://doi.org/10.1177/0284185116687172
- Kanona H, Rana I, Offiah C. Importance of a dedicated neuroradiologist in reporting high-resolution computed tomography for otosclerosis: a retrospective comparison study of 40 patients. J Laryngol Otol. 2017;131:492-496. doi:https://doi.org/10.1017/S0022215117000561
- Ukkola-Pons E, Ayache D, Pons Y. Oval window niche height: quantitative evaluation with CT before stapes surgery for otosclerosis. AJNR Am J Neuroradiol. 2013;34:1082-1085. doi:https://doi.org/10.3174/ajnr.A3354
- Virk J, Singh A, Lingam R. The role of imaging in the diagnosis and management of otosclerosis. Otol Neurotol. 2013;34:E55-E60. doi:https://doi.org/10.1097/MAO.0b013e318298ac96
- Ziyeh S, Berlis A, Ross U. MRI of active otosclerosis. Neuroradiology. 1997;39:453-457. doi:https://doi.org/10.1007/s002340050445
- Steenerson K, Crane B, Minor L. Superior semicircular canal dehiscence syndrome. Semin Neurol. 2020;40:151-159. doi:https://doi.org/10.1055/s-0039-3402738
- Sioshansi P, Drury E, Tu N. Outcomes of stapedotomy in patients with concomitant otosclerosis and superior semicircular canal dehiscence: should a radiographic third-window be a contraindication to stapes surgery?. Otol Neurotol. 2022;43:165-169. doi:https://doi.org/10.1097/MAO.0000000000003429
- McClellan J, Nguyen A, Hamilton B. Stapes surgery outcomes in patients with concurrent otosclerosis and superior semicircular canal dehiscence. Otol Neurotol. 2020;41:912-915. doi:https://doi.org/10.1097/MAO.0000000000002673
- Fernandez I, Villari D, Botti C. Endoscopic revision stapes surgery: surgical findings and outcomes. Eur Arch Otorhinolaryngol. 2019;276:703-710. doi:https://doi.org/10.1007/s00405-019-05280-4
- Sennaroğlu L, Bajin M. Classification and current management of inner ear malformations. Balkan Med J. 2017;34:397-411. doi:https://doi.org/10.4274/balkanmedj.2017.0367
- Whetstone J, Nguyen A, Nguyen-Huynh A. Surgical and clinical confirmation of temporal bone CT findings in patients with otosclerosis with failed stapes surgery. AJNR Am J Neuroradiol. 2014;35:1195-1201. doi:https://doi.org/10.3174/ajnr.A3829
- Walker B, Thorwarth R, Stull L. Incidence of concomitant semicircular canal dehiscence with otosclerosis. Otology Neurotol Open. 2022;2. doi:https://doi.org/10.1097/ONO.0000000000000012
- Williams M, Ayache D, Elmaleh M. Helical CT findings in patients who have undergone stapes surgery for otosclerosis. AJR Am J Roentgenol. 2000;174:387-392. doi:https://doi.org/10.2214/ajr.174.2.1740387
- Williams M, Ayache D. Imaging of the postoperative middle ear. Eur Radiol. 2004;14:482-495. doi:https://doi.org/10.1007/s00330-003-2198-8
- Rangheard A, Marsot-Dupuch K, Mark A. Postoperative complications in otospongiosis: usefulness of MR imaging. AJNR Am J Neuroradiol. 2001;22:1171-1178.
- Warren F, Riggs S, Wiggins R. Computed tomographic imaging of stapes implants. Otol Neurotol. 2008;29:586-592. doi:https://doi.org/10.1097/MAO.0b013e3181758e96
- Bozzato A, Struffert T, Hertel V. Analysis of the accuracy of high-resolution computed tomography techniques for the measurement of stapes prostheses. Eur Radiol. 2010;20:566-571. doi:https://doi.org/10.1007/s00330-009-1582-4
- Hahn Y, Diaz R, Hartman J. Assessing stapes piston position using computed tomography: a cadaveric study. Otol Neurotol. 2009;30:223-230. doi:https://doi.org/10.1097/MAO.0b013e31818de5cd
- Doerfer K, Tu N, Sioshansi P. Preoperative evaluation of otosclerosis: a national survey of otologists. Otol Neurotol. 2022;43:E963-E968. doi:https://doi.org/10.1097/MAO.0000000000003669
- Akazawa Y, Ganaha A, Higa T. Measurement of stapes footplate thickness in otosclerosis by ultra-high-resolution computed tomography. Acta Otolaryngol. 2020;140:899-903. doi:https://doi.org/10.1080/00016489.2020.1788225
- Miracle A, Mukherji S. Conebeam CT of the head and neck, part 1: physical principles. AJNR Am J Neuroradiol. 2009;30:1088-1095. doi:https://doi.org/10.3174/ajnr.A1653
- Miracle A, Mukherji S. Conebeam CT of the head and neck, part 2: clinical applications. AJNR Am J Neuroradiol. 2009;30:1285-1292. doi:https://doi.org/10.3174/ajnr.A1654
- Small B. Cone beam computed tomography. Gen Dent. 2007;55:179-181.
- Molteni R. Prospects and challenges of rendering tissue density in Hounsfield units for cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:105-119. doi:https://doi.org/10.1016/j.oooo.2013.04.013
- Redfors Y, Grondahl H, Hellgren J. Otosclerosis: anatomy and pathology in the temporal bone assessed by multi-slice and cone-beam CT. Otol Neurotol. 2012;33:922-927. doi:https://doi.org/10.1097/MAO.0b013e318259b38c
- Liktor B, Revesz P, Csomor P. Diagnostic value of cone-beam CT in histologically confirmed otosclerosis. Eur Arch Otorhinolaryngol. 2014;271:2131-2138. doi:https://doi.org/10.1007/s00405-013-2702-y
- Révész P, Liktor B, Liktor B. Comparative analysis of preoperative diagnostic values of HRCT and CBCT in patients with histologically diagnosed otosclerotic stapes footplates. Eur Arch Otorhinolaryngol. 2016;273:63-72. doi:https://doi.org/10.1007/s00405-015-3490-3
- Deng F, Touska P, Reinshagen K. Diagnostic performance of conebeam CT pixel values in active fenestral otosclerosis. AJNR Am J Neuroradiol. 2021;42:1667-1670. doi:https://doi.org/10.3174/ajnr.A7192
- Xu N, Ding H, Tang R. Comparative study of the sensitivity of ultra-high-resolution CT and high-resolution CT in the diagnosis of isolated fenestral otosclerosis. Insights Imaging. 2023;14. doi:https://doi.org/10.1186/s13244-023-01562-y
- Willemink M, Persson M, Pourmorteza A. Photon-counting CT: technical principles and clinical prospects. Radiology. 2018;289:293-312. doi:https://doi.org/10.1148/radiol.2018172656
- Pourmorteza A, Symons R, Henning A. Dose efficiency of quarter-millimeter photon-counting computed tomography: first-in-human results. Invest Radiol. 2018;53:365-372. doi:https://doi.org/10.1097/RLI.0000000000000463
- Danielsson M, Persson M, Sjölin M. Photon-counting x-ray detectors for CT. Phys Med Biol. 2021;66. doi:https://doi.org/10.1088/1361-6560/abc5a5
- Mannil M, Hickethier T, von Spiczak J. Photon-counting CT: high-resolution imaging of coronary stents. Invest Radiol. 2018;53:143-149. doi:https://doi.org/10.1097/RLI.0000000000000420
- Do T, Sawall S, Heinze S. A semi-automated quantitative comparison of metal artifact reduction in photon-counting computed tomography by energy-selective thresholding. Sci Rep. 2020;10. doi:https://doi.org/10.1038/s41598-020-77904-3
- Zhou W, Bartlett D, Diehn F. Reduction of metal artifacts and improvement in dose efficiency using photon-counting detector computed tomography and tin filtration. Invest Radiol. 2019;54:204-211. doi:https://doi.org/10.1097/RLI.0000000000000535
- Zhou W, Lane J, Carlson M. Comparison of a photon-counting-detector CT with an energy-integrating-detector CT for temporal bone imaging: a cadaveric study. AJNR Am J Neuroradiol. 2018;39:1733-1738. doi:https://doi.org/10.3174/ajnr.A5768
- Benson J, Rajendran K, Lane J. A new frontier in temporal bone imaging: photon-counting detector CT demonstrates superior visualization of critical anatomic structures at reduced radiation dose. AJNR Am J Neuroradiol. 2022;43:579-584. doi:https://doi.org/10.3174/ajnr.A7452
- Goh J, Chan L, Tan T. MRI of cochlear otosclerosis. Br J Radiol. 2002;75:502-505. doi:https://doi.org/10.1259/bjr.75.894.750502
- Purohit B, Op de Beeck K, Hermans R. Role of MRI as first-line modality in the detection of previously undiagnosed otosclerosis: a single tertiary institute experience. Insights Imaging. 2020;11. doi:https://doi.org/10.1186/s13244-020-00878-3
- Vicente A de O, Chandrasekhar S, Yamashita H. Magnetic resonance imaging in the evaluation of clinical treatment of otospongiosis: a pilot study. Otolaryngol Head Neck Surg. 2015;152:1119-1126. doi:https://doi.org/10.1177/0194599815574698
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1282 times
- PDF downloaded - 244 times



