Reviews
Vol. 45: Issue 3 (Suppl. 1) - June 2025
Update on stapes surgery
Abstract
Stapes surgery has significantly evolved from the early identification of stapes fixation as a cause of hearing loss in the 18th century to advanced modern techniques. This narrative review examines the historical development and contemporary advancements in stapes surgery, focusing on all the critical aspects of surgical procedures: from patient positioning, to microscopic versus endoscopic visualization, type of anaesthesia, characteristics of prosthesis, and different surgical techniques. A further analysis of special conditions has been made.
Introduction
The history of stapes surgery is a demonstration that the relentless pursuit of medical innovation has significantly advanced our understanding and treatment of hearing loss (HL). Stapes fixation causing HL was first identified by Antonio Maria Valsalva in 1704. In 1841, Toynbee’s dissection of 1,659 temporal bones found 39 cases of stapes fixation, linking it to deafness. By 1893, Adam Politzer’s histological studies indicated otosclerosis as the cause 1.
Johannes Kessel described stapes surgery in 1876, attributing HL to increased inner ear fluid pressure. His methods included mobilising or removing the stapes, with mixed success and some risks like labyrinthitis and meningitis 2. His techniques were criticised and deemed dangerous by the early 20th century 1. Surgeons then shifted to “third-window” fenestration techniques, fully established by Jenkins in 1913 with contributions from Lempert, who simplified the procedure 3,4. Samuel Rosen reintroduced stapes mobilisation in the mid-20th century, achieving immediate but often temporary hearing improvement 5.
John Shea revolutionised stapes surgery in 1956 by successfully using a Teflon prosthesis to replace the stapes after a complete stapedectomy, with the interposition of autologous material, usually constituted by a vein or perichondrium 6. His technique, initially considered dangerous, became standard by the 1960s 7. Schuknecht later developed a steel-wire prosthesis in 1960, while Plester proposed a partial footplate removal method, leading to further advancements in stapedectomy procedures 8. This advancement paved the way for modern stapes surgery, featuring the use of piston prostheses and the creation of small holes in the footplate.
This narrative review aims to provide an overview of the evolution of surgical techniques in stapes surgery, analysing each technical aspect in detail, including the position of the patient during surgery, the instruments used for visualising the middle ear, type of anaesthesia administered, methods for removing the stapes’ superstructure, techniques for creating a hole in the footplate, and the characteristics of the prosthetic piston. Additionally, the review will include a dedicated section on stapes surgery in special conditions.
Surgical procedures
Due to the intrinsic danger of sensorineural hearing damage and the higher rate of complications 7,9, stapedectomy is nowadays outdated and stapedotomy is the preferred surgical treatment for fenestral otosclerosis with good cochlear reserve.
Traditionally, modern stapes surgery through stapedotomy involves the use of a microscope and a transcanal approach under local anaesthesia. The procedure includes preparing the tympanomeatal flap, removing part of the bony frame to visualise the ossicular chain (stapes, incus, second portion of the facial nerve, pyramidal eminence), removing the stapes superstructure, preparing the footplate hole, placing the piston prosthesis between the incus and the footplate hole, and finally repositioning and packing the tympanomeatal flap.
For an optimal surgical result, it is crucial to carefully analyse every step and aspect of the procedure.
Patient position
Traditionally, the patient should be supine on the operating table with the head hyperextended and rotated to expose the ear to be operated (Fig. 1). The surgeon stands very close to the patient, resting their wrists on the patient’s head to minimize hand movement errors. Even in the paper by Mantokoudis and colleagues that recently revised classical microscopic transcanal approach in stapes surgery, the positioning of the patient is considered a crucial step in the surgical procedure 10. In our opinion, furthermore, the operating table should be placed as low as possible and tilted in a reverse Trendelenburg position, with the headrest angle maximally reclined.
Instruments to visualise: microscope and endoscope
The first documented use of a binocular microscope in otologic surgery is attributed to Gunnar Holmgren in 1922. However, Emilio De Rossi is often cited as the first to use a binocular magnification system in 1869, although it consisted of a single lens rather than a true binocular microscope. Carl Olof Nylen was the first to use a monocular microscope in 1921, which was quickly replaced by Holmgren’s binocular model 11.
Afterwards, the evolution of the binocular operating microscope in otologic surgery marked a significant advancement, enabling a less invasive transcanal approach to the middle ear. Previously, more invasive methods like endoaural incisions or retroauricular approaches were common. Although it limits the working space, a transcanal microscopic approach provides binocular vision into the middle ear without needing an extensive skin incision, thus reducing postoperative pain and bleeding. Moreover, this minimally invasive technique avoids complications such as scar tissue formation, hypoaesthesia of the auricle, and pinna protrusion. However, its limited field of view can necessitate additional procedures, such as endoaural incisions and drilling of the auditory canal or scutum, and frequent repositioning of the surgeon and patient 10,12-14 (Fig. 2).
One alternative to using a microscope is endoscopy. Endoscopic surgery for otosclerosis offers a significant advantage with its wide-angle view, reducing the need for scutum removal and enhancing exposure for teaching and training 14,15. This wide-angle view allows for closer and more precise visualisation of the footplate, with no or minimal bone removal and need to manipulate the chorda tympani nerve 12-14. Despite these benefits, there is currently no objective method to quantify the improved visibility provided by the endoscope, making this advantage largely subjective and based on the individual surgeon’s experience. Moreover, endoscopic surgery has its drawbacks, including reduced depth perception due to its two-dimensional view and the need to operate with one hand, which can complicate the procedure and increase the learning curve 13. Usually, surgeons familiar with using the microscope prefer to continue with it for these reasons. Regarding the size of the endoscopes, 4 mm nasal endoscopes were initially used, but 3 mm endoscopes have become standard in otology. Studies have not demonstrated a clear superiority of narrower endoscopes, as hearing outcomes and complication rates are similar with both sizes, despite reports of better visibility with smaller endoscopes 16.
The introduction of endoscopes did not alter the fundamental surgical techniques but provided an alternative access route, with hearing outcomes remaining comparable to those of microscopic surgery. This was confirmed by systematic reviews showing no significant difference in air-bone gap closure across frequencies 13,14,17,18. No significant difference in complications such as chorda tympani nerve injury, dysgeusia, or residual perforation was found between endoscopic and microscopic procedures, although some studies suggested lower chorda tympani injury rates with endoscopes due to less scutum removal 18. Despite some studies indicating higher complication rates with endoscopic surgery, also due to thermal injury, recent reviews have not found significant differences between the two approaches 13,19. As assessed in the study by Molinari and collaborators, surgeon’s experience is also a critical factor, potentially biasing outcomes, for instance leading to reduced operating times if an experienced endoscopic surgeon carries out the procedure 18.
In conclusion, while both microscopic and endoscopic approaches to stapes surgery have their pros and cons, neither can be definitively considered superior. The choice of surgical method should be based on the surgeon’s expertise, training, and the availability of appropriate tools to ensure the safe execution of stapedotomy or stapedectomy.
Instruments to visualise: speculum and other approaches
In microscopic approaches, the choice of auricular speculum is crucially important. It is first useful to assess the diameter of the canal by placing an auricular speculum with a diameter of 5 mm, in order to understand whether it is reasonable to carry out a transcanal approach or if another route is indicated. In fact, a classical transcanal microscopic approach could be challenging if a narrow external auditory canal does not accept at least a 5 mm diameter speculum 20.
According to Mantokoudis and collaborators, the largest speculum usable according to the size of the external auditory canal and allowing visualisation of the hammer handle anteriorly and the posterior wall of the ear canal posteriorly should be preferred 10. The instruments must be held like a pencil with the first three fingertips, stabilising the hand on the speculum or the patient’s head with the other two fingers 10 (Fig. 3). Even if not all the surgeons use it, a speculum holder – consisting of a mobile extension mounted on the operating table – can be used to fix the speculum thus simplifying the surgeon’s bimanual actions 10.
An alternative way to stapes surgery is the endoaural approach, especially by an intertragal incision. This method allows surgeons to bypass the need for an auricular speculum. This type of approach can help bimanual surgery, offers a clear view of the middle ear, and can be advantageous in cases involving narrow external ear canals 21.
Type of anaesthesia
Stapedotomy can be performed both under local and general anaesthesia. Some surgeons prefer to use local anaesthesia with or without sedation to monitor auditory and vestibular responses during surgery, while others prefer general anaesthesia for the patient’s comfort.
In a 2008 study, Vital and collaborators found a higher incidence of profound HL in patients under general anaesthesia (1.8%) compared to local anaesthesia (0%) 22. On the other hand, a systematic review of 417 procedures showed no significant differences in postoperative outcomes between anaesthesia methods 23.
In our opinion, local anaesthesia is preferable, since it allows the surgeon to monitor the patient’s reactions during surgical manipulation of middle ear structures. Typically, 2% lidocaine with 1:100,000 epinephrine is used for its quick anaesthetic effect. About 10 mL is injected at various sites, not exceeding 7 mg/kg. Infiltration starts in the retroauricular region, blocking nerves to the outer ear, and continues between the tragus and helix, and in the posterior external auditory canal. The association with adrenaline can further reduce bleeding and improve haemostasis 24.
Tympanomeatal flap harvesting and scutum removal
The tympanomeatal flap should be U-shaped and created from 6 to 12 o’clock, encompassing the upper, rear, and lower walls of the ear canal 10,25. The skin should be incised a few mm distally from the end of the speculum. The skin of the flap is then detached up to the posterior bony annular edge 10,25. This step may cause bleeding, which can be controlled using an adrenaline-soaked absorbable gelatin sponge 10. Accessing the tympanic cavity and detaching the tympanic membrane is best done at the posterosuperior quadrant, referred to as Rivino’s engraving, where the fibrous annulus of the tympanic membrane is less adherent to the bony edge. Once the tympanic membrane has been detached from the bony rim, the tympanomeatal flap is folded anteriorly 25. If the surrounding bracket and structures are not sufficiently exposed, the back-top of the ear canal can be removed using a bone curette or a 2 mm low-speed diamond drill 10. The structures that must be visualised before proceeding to the next step are the bracket, the long process of the incus, the tympanic part of the facial nerve, and the pyramidal eminence with the tendon of the stapedius muscle 10.
Removing the superstructure of the stapes
The traditional stapedotomy procedure involves removing the superstructure of the stapes before creating an opening into the footplate and inserting the prosthesis. In 1987, Fisch proposed to reverse these steps during stapedotomy to reduce the risk of a floating footplate, inner ear damage, and dislocation of the ossicular chain 9. Instead of removing the stapes superstructure first, Fisch proposed performing the fenestration first and then placing the prosthesis, keeping both the incudostapedial joint and stapedius tendon intact. Once the prosthesis is secured, the stapes and the lenticular process of the incus are separated, the stapes crura is fractured, the stapedius tendon is cut, and the superstructure is removed. This reversal of steps decreases the exposure time of the vestibule, minimises blood entry, and reduces the need for manipulation and the risk of inner ear injury. An additional benefit of Fisch’s reversed steps stapedotomy is the increased stability of the ossicular chain, making it easier to position the piston on the long process of the incus 9.
In 2008, Fiorino and Barbieri described a reversal steps stapedotomy technique with early removal of the posterior crus. In our opinion, this technique can provide better visualisation of the stapes footplate and, simultaneously, by retaining the anterior crus of the stapes until after the prosthesis is secured, the technique maintains the stability of the ossicular chain throughout the procedure 26.
Performing the footplate hole
The next surgical step is creating the footplate hole. Fenestration techniques in stapedotomy have advanced significantly with the introduction of microdrills and lasers, moving beyond conventional manual drills (Trefine) 10. Historically, manual drills were widely used and favoured due to their familiarity among surgeons and their simplicity, especially when dealing with thin footplates. The advent of microdrills marked a certain improvement in stapedotomy, particularly in shortening the operation time, especially when the footplate is very thick. Microdrills, equipped with small diamond burrs of 0.6-0.7 mm in diameter, operate with low noise intensity and low torque, making them a safer option for footplate drilling without causing significant acoustic trauma. This was shown in numerous recent studies 27-31. Studies have further demonstrated that microdrills can create precise, round holes that match the size of the prosthesis, reducing the risk of fistula and granulation tissue formation 29, as well as that they result in better audiologic outcomes compared to manual drills 30. However, despite their benefits, microdrills also present certain risks, such as the possibility of advancing into the vestibule and causing sensorineural HL and vertigo 27. Additionally, their use in endoscopic surgery is complicated by reduced depth perception, leading to longer operation times 32.
The introduction of lasers in stapedotomy in the 1980s aimed to minimise mechanical manipulation of the footplate and inner ear 33. Lasers allow for soft touch or no-touch-at-all perforation of the footplate, thereby reducing the risk of mechanical trauma 34,35.
Various types of lasers, including argon, diode, KTP, thulium, and CO2, offer distinct characteristics that cater to different surgical needs. Laser technology provides high precision, a bloodless surgical field, and the ability to cut, vaporise, and coagulate tissue using thermal energy. Despite these advantages, lasers also pose potential risks, such as overheating of the perilymph, acoustic trauma, and other complications specific to each laser type. Furthermore, the high cost and need for specialised training and equipment can be limiting factors 35.
Comparative studies have yielded mixed results regarding the superiority of microdrills and other conventional methods over lasers. On the other hand, research comparing laser techniques with conventional methods showed varied outcomes. Silverstein and colleagues reported improved audiologic outcomes with the KTP laser, but noted prolonged dizziness and instability in some patients 36. Similarly, Arnoldner and collaborators found that while hearing results were comparable, the incidence of perilymphatic fistula was higher in laser-assisted surgeries 37. Other studies, such as those by Pauli and colleagues and Altamami and colleagues, found no significant differences in hearing thresholds between different surgical techniques involving the use of lasers 38,39. A systematic review by Wegner and collaborators in 2013 reported no evidence that laser techniques were superior to others in postoperative hearing outcomes, even if the rates of footplate fracture and sensorineural hearing damage seemed to be increased with the use of perforators or microdrills 40.
Moreover, even if each type of laser used in stapedotomy has unique characteristics, there is no conclusive evidence proving the clinical superiority of one laser type over another or over traditional techniques 40. In the systematic review by Wegner and colleagues, it also emerged that many studies suffer from biases, small sample sizes, and methodological inconsistencies, making it difficult to draw definitive conclusions 40. In the absence of robust evidence, the choice of operative technique often depends on the surgeon’s preference, experience, and specific clinical circumstances.
Characteristic of the piston
The rigidity of the annular ligament constitutes 90% of the total impedance in the human middle ear at lower frequencies, thereby playing a crucial role in sound transmission, particularly for speech frequencies. The solid collagen fibres of the annular ligament significantly influence the amplitude of stapedial vibrations during low-frequency acoustic stimulation. The sound pressure at the cochlear entrance correlates directly with the volume velocity of the stapes, which is defined by the product of the area and amplitude of the vibrating footplate. With an area of approximately 3.2 mm2, the footplate vibrates at amplitudes of only a few nanometers to displace a sufficient volume of liquid to transmit sound pressure into the cochlea under physiological conditions 41,42. Replacing an otosclerotic stapes with a piston prosthesis eliminates the annular ligament as the primary impedance factor in the middle ear, allowing the ossicles to vibrate with greater amplitudes at equivalent sound pressures at the tympanic membrane. Consequently, a piston prosthesis with a smaller contact area, such as a 0.4 mm piston, can vibrate with a much larger amplitude at equivalent sound pressures, compensating for its smaller surface area 41 (Fig. 4). However, reducing the diameter of a piston is limited by the vibrational capacity of the tympanic membrane and the ossicles. Animal experiments suggest that the maximal vibrational amplitude of sound-transmitting structures is achieved with a piston diameter smaller than 0.4 mm, and volume velocity decreases with smaller diameters 42. Clinical findings support this lower limit, with Grolman et al. reporting decreased sound transmission, especially below 1 kHz, for a 0.3 mm compared to a 0.4 mm piston 43. Pistons with diameters of 0.4 mm and greater generally exhibit similar sound transmission properties if the vibrational capacity of the middle ear structures remains unrestricted. However, literature on the acoustic results following stapedoplasty with various piston diameters is inconsistent. For instance, Fisch 9 and Shabana et al. 44 found no significant difference in hearing outcomes at speech frequencies between 0.4 and 0.6 mm pistons. In contrast, as reported in Hüttenbrink review, a significant advantage for the larger diameter at 500 Hz was observed, although both diameters performed equally at higher frequencies 41. Other studies, such as those by Häusler, and Coletti et al., have reported varying results, indicating better performance at different frequencies depending on the piston diameter used 41,45. Mathematical models have also been used to predict the impact of piston diameter on sound transmission, generally suggesting that larger diameters may offer advantages. However, these models often rely on experimental data with inherent methodological discrepancies, leading to varying results. These studies must be carefully interpreted, as inaccuracies in parameter estimation can distort results. The vibrating area in stapedoplasty is not solely defined by the piston’s diameter, as the surrounding connective tissue membrane also contributes to sound transmission (Fig. 4). Clinical audiology, in fact, typically does not register large deficits after stapedoplasty with a 0.4 mm piston, despite theoretical predictions of significant losses. Fucci et al. found that 0.4 and 0.6 mm steel pistons inserted into identically sized fenestrations performed equally well 46.
Moreover, many studies comparing acoustic outcomes with different piston diameters do not specify the size of the footplate fenestra, and most surgeons aim to perforate the footplate slightly larger than the piston diameter to minimise inner ear trauma. The area that vibrates the labyrinthine fluids is actually determined by the size of the footplate hole, not the diameter of the piston 47, and this aspect could lead to bias.
The material of the piston may also impact on the acoustic performance. Heavier prostheses, like those made from steel or gold, tend to perform better at lower frequencies, while lighter materials, such as Teflon, transmit sound more effectively at higher frequencies. This effect is due to shifts in the resonance frequency of the reconstructed middle ear 43,48,49. However, the overall influence of piston mass on sound transmission is relatively minor, as even significant increases in mass result in less than a 10 dB reduction in transmission. This minor influence was demonstrated in early animal experiments 50 and later supported by mathematical models 47 and temporal bone studies 51,52, although some of these studies reported contradictory data 41. In any case, surgeons currently use only lightweight prostheses. Therefore, the only fundamental principle behind prostheses used in otosclerosis surgery is to create a secure connection between the mobile long process of the incus and the perilymph in the oval window. Since Shea and Treace first introduced a Teflon stapes replica in 1956 3, many types of stapes prostheses have been developed. Advancements in surgical materials, including those with greater biocompatibility, have played a key role in the development of new prostheses. Innovations like shape-memory prostheses were also introduced 53, as well as non-ferromagnetic implants for magnetic resonance compatibility 54.
Fritsch and Naumann classified stapedectomy prostheses into 4 categories: wire loop, piston, bucket, and homemade 54. Commercial prostheses (wire loop, piston, and bucket) typically consist of 3 parts: the incus attachment end, the shaft, and the oval window attachment base 54. Innovations in these areas have aimed to prevent complications like incus necrosis, often caused by ischaemia from pressure during crimping or foreign body reactions, as well as dislocation, and maximise hearing outcomes as well as facilitate the surgeon’s work during the procedure 54.
Materials used for stapes prostheses include stainless steel, platinum, titanium, nitinol, and Teflon. Stainless steel is chosen by some surgeons for its rigidity, shape retention, and malleability. Platinum, although malleable, has been associated with higher incus necrosis rates, possibly due to local toxicity 54. Titanium is lightweight, rigid, and biocompatible, forming a protective titanium oxide layer upon oxidation, reducing granulation and scar tissue formation. Nitinol prostheses, made in a nickel-titanium alloy, which revert to their original shape when heated, offer advantages in fixation but its nickel content may raise concerns about biocompatibility, even if some studies seem to reject this hypothesis 55. Teflon, a common material, is chemically stable, malleable, and resistant to corrosion, with a ‘memory effect’ that minimises incus necrosis due to ischaemia. Comparative studies have shown no significant differences in audiologic outcomes between various materials 49,56,57, or in the rate of complications 57.
Some prostheses come in predefined sizes, while others can be trimmed to fit during surgery. Shaft diameter can also vary. Some studies suggest better hearing results with larger diameter prostheses, although the choice often depends on the surgeon’s skill and the specific case 58. As described by Fisch in 1994 59, and confirmed by the review from Hüttenbrink in 2003 41, the length of the stapes prosthesis should be calibrated so that approximately 0.5 mm of the prosthesis extends into the vestibule. This length should help to prevent the prosthesis from dislocating during lateral movements of the incus, such as those caused by sneezing or during a Valsalva manoeuvre. Additionally, it safeguards the delicate structures of the inner ear, particularly the utriculus and sacculus, from damage when the incus moves medially, as might occur during an increase in atmospheric pressure.
Crimping of the prosthesis
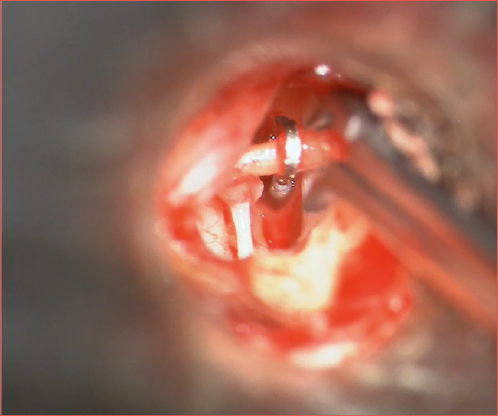
One of the critical steps during stapes surgery is the coupling or crimping of the prosthesis onto the incus (Cover figure). Improper crimping, whether too tight, too loose, or causing mechanical trauma during the process, can potentially lead to incus erosion 60,61. Furthermore, both experimental and clinical studies have demonstrated that the quality of crimping is directly associated with postoperative hearing outcomes 62. Consequently, various prostheses have been developed with different crimping methods. Current stapes prostheses can be categorised into self-crimping and manually crimped types. Self-crimping options eliminate the need for manual crimping and its potential complications, even if the incus end loop diameter of these types of prosthesis is often predefined and in our opinion cannot always adequately fit all subjects.
Initially, some prostheses, such as bucket-handle and cup prostheses, as well as Teflon self-crimping prostheses, were designed to couple with the incus without additional crimping. More recently, shape-memory heat-crimping prostheses made from a nickel-titanium alloy known as nitinol have been introduced 63. These prostheses are crimped by applying heat via a laser or bipolar forceps, which activate the shape-memory properties of nitinol, causing the prosthesis loop to close. Using a laser-crimped nitinol prosthesis avoids direct contact with the prosthesis or the incus, thereby preventing trauma that may occur with manual crimping 64.
Even if manual crimping is technically more challenging, heat crimping can also have drawbacks, such as the potential for vaporisation of blood vessels, leading to necrosis of the long process of the incus 55. The effect of various crimping methods on hearing outcomes after stapes surgery has been the focus of numerous studies, although the quality of these papers is relatively low 63,65-68. Most of these studies are small and do not reach statistical significance. Among those that do, the findings consistently favour heat-crimped prostheses over manually crimped ones, and manual crimping over no crimping. None of the crimping methods appear to increase the incidence of adverse events 62. Until further high-quality studies with sufficient follow-up duration are conducted to confirm these findings, it is reasonable for surgeons to use the type of prosthesis and crimping method with which they feel most comfortable 62.
Sealing of the footplate
The necessity of sealing the oval window is debated, with some studies suggesting that a well-executed technique is more important than the use of a sealant for preventing perilymphatic fistula 69. However, some surgeons choose not to seal the oval window at all, as the additional step may complicate an otherwise straightforward procedure 69.
Different materials for sealing the oval window in stapes surgery exist, both autologous and heterologous, all with advantages and drawbacks. The principal autologous are fat, vein, fascia, perichondrium, and blood clot. The most widely used are heterologous represented by hyaluronic acid, gelatin sponge, and the acellular porcine-derived matrix 69.
Fat is seen as a practical and effective option for sealing, being both cost-effective and stable over time, with outcomes similar to other autologous tissues like vein and fascia. The use of vein grafts, which are compatible with middle ear mucosa and stable over the long term, is well-established. Veins are traditionally harvested from the wrist or hand, but using the superficial temporal vein offers better cosmetic results and convenience by utilising the same operative site 69. Gelfoam, introduced by House for stapes surgery 70, is easy to handle and widely available. It does not require an additional surgical incision, thus reducing surgical time and associated risks. However, it can cause adhesions and fibrosis, especially in inflamed or exposed mucosa. Some studies have shown no significant difference between using Gelfoam and not sealing at all, leading some practitioners to stop using it. Autologous materials such as perichondrium and fascia are also cost-effective and compatible with the middle ear, although harvesting these tissues can extend the duration of surgery 69. There are concerns about the chondrogenic potential of perichondrium, but these can be mitigated with proper handling. Scarpa et al. found that hearing outcomes and vestibular complication rates are similar regardless of the sealant used, suggesting that the choice of material should be based on the surgeon’s preference, as no clear evidence favours one material over another 69. The authors of the present review drop only a blood clot to seal the footplate.
Special conditions
Age – Stapes surgery is a safe and effective treatment for any age, once stapes fixation is confirmed. There is not a superior 71 or inferior 72 age limit for undergoing such a procedure.
Chefs and sommeliers – Stapes surgery should be carefully counselled in chefs and sommeliers due to the risk of permanent taste disorders. Alternative treatments like hearing aids should be considered. If surgery is chosen, patients must be informed of the potential loss of work function 73.
Aviation – Thiringer and Arriaga studied 16 US Air Force aircrew members who returned to flight duty post-stapedectomy without complications 74. Katzav et al. reported similar success in Israeli Air Force pilots 75. While military pilots in Brazil are deemed unfit post-surgery, civil aviation does not restrict stapedectomy, although those with permanent vestibular disorders cannot be certified 76.
Diving – Scuba diving may increase the risk of perilymphatic fistula and prosthesis displacement. Studies show no significant risk, but 54.3% of surgeons recommend permanent diving restrictions after stapes surgery. Despite some postoperative otologic symptoms, no strong evidence links these to diving 76,77.
Conclusions
This narrative review on stapes surgery provides an overview of this surgical procedure, highlighting both historical and modern advancements. Below are some personal considerations based on the comprehensive content presented. The historical evolution of stapes surgery is a testament to the relentless pursuit of medical innovation. Starting from Valsalva’s initial identification of stapes fixation to Shea’s introduction of the Teflon prosthesis, each advancement has been built on the shoulders of previous discoveries. The shift from microscopic to endoscopic techniques represents an appreciable technological step. Endoscopy offers certain advantages, but also has some limitations, such as reduced depth perception and the need for one-handed operation.
The choice of technique for creating the footplate hole, whether using manual perforators, microdrills or lasers, remains a topic of debate. While microdrills offer precision and reduced operation times, they carry risks such as vestibular penetration. On the other hand, lasers minimise mechanical trauma but present challenges like potential overheating and higher costs. The variety of prosthetic designs, dimensions and materials reflects the complexity and individuality of medical practice. The fact that no single type or material has emerged as superior underscores the importance of a personalised approach. Surgeons must weigh the benefits and drawbacks of each type of prosthesis based on the specific patient characteristics, as well as their own surgical experience. The advancements in design of the prosthesis, particularly the move towards lighter and more biocompatible materials, and the possibility of self-crimping technologies, have not only enhanced the functionality of this surgical procedure, but also reduced the risk of complications. The ongoing evolution of stapes surgery is a reminder that medical practice is never static, and that continuous improvement is vital for achieving the best possible patient outcomes. It is also important to underline the importance of historical knowledge, technological innovation, and ongoing research in the field, which is essential for practitioners to provide the highest standard of care in otologic surgery.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
LB, FL, ADV: conceived the initial idea for the narrative review and developed the structure of the article; FF, SB: performed the literature review and critically analyzed the sources. All authors contributed significantly to drafting the manuscript, revising it for important intellectual content, and approving the final version to be submitted. Each author has read and agreed to the published version of the manuscript.
Ethical consideration
Not applicable.
History
Received: November 25, 2024
Accepted: April 7, 2025
Figures and tables
Figure 1. Patient positioning for stapes surgery: A) the patient should be positioned supine on the operating table with the head hyperextended and rotated to expose the ear to be operated; B) to obtain a better exposure, the table can be tilted on the frontal (coronal) plane.
Figure 2. Microscope vision during transcanal stapes surgery.
Figure 3. Hand and speculum position for transcanal stapes surgery. The instruments must be held like a pencil with the first three fingertips, stabilising the hand on the speculum or the patient’s head with the other two fingers.
Figure 4. Vibration model of a piston stapes prosthesis.
References
- Nazarian R, McElveen JT, Eshraghi AA. History of otosclerosis and stapes surgery. Otolaryngol Clin North Am 2018;51:275-290. https://doi.org/10.1016/j.otc.2017.11.003
- McElveen JT, Kutz JW. Controversies in the evaluation and management of otosclerosis. Otolaryngol Clin North Am 2018;51:487-499. https://doi.org/10.1016/j.otc.2017.11.017
- Shea JJ. A personal history of stapedectomy. Am J Otol 1998;19:S2-S12.
- Beales PH. Otosclerosis – Past and present. J R Soc Med 1979;72:553-561. https://doi.org/10.1177/014107687907200803
- Pietruski J. Samuel Rosen (1897-1981): the originator of stapes mobilization. Otolaryngol Pol 1999;53:739-742.
- Shea JJ. Fenestration of the oval window. Ann Otol Rhinol Laryngol 1958;67:932-951. https://doi.org/10.1001/archotol.1960.03770020129019
- Cajade Frías J, Labella Caballero T. Historical analysis of otosclerosis surgery. Acta Otorrinolaringol Esp 1999;50:591-596.
- Schuknecht HF, McGee TM, Colman BH. Stapedectomy. Ann Otol Rhinol Laryngol 1960;69:597-609. https://doi.org/10.1177/000348946006900221
- Fisch U. Stapedotomy versus stapedectomy. Am J Otol 1982;4:112-117.
- Mantokoudis G, Weder S, Anschuetz L, et al. The microscopic transcanal approach in stapes surgery revisited. J Vis Exp 2022;180. https://doi.org/10.3791/63011-v
- Mudry A. The history of the microscope for use in ear surgery. Am J Otol 2000;21:877-886.
- Tarabichi M. Endoscopic middle ear surgery. Ann Otol Rhinol Laryngol 1999;108:39-46. https://doi.org/10.1177/000348949910800106
- Koukkoullis A, Toth I, Gede N, et al. Endoscopic versus microscopic stapes surgery outcomes: a meta-analysis and systematic review. Laryngoscope 2020;130:2019-2027. https://doi.org/10.1002/lary.28353
- Lucidi D, Molinari G, Reale M, et al. Functional results and learning curve of endoscopic stapes surgery: a 10-year experience. Laryngoscope 2021;131:885-891. https://doi.org/10.1002/lary.28943
- Iannella G, Marcotullio D, Re M, et al. Endoscopic vs microscopic approach in stapes surgery: advantages in the middle ear structures visualization and trainee’s point of view. J Int Adv Otol 2017;13:14-20. https://doi.org/10.5152/iao.2017.3322
- Pradhan P, Preetam C. Endoscopic stapedotomy: a comparison between 4 mm and 3 mm nasal endoscope. Eur Arch Otorhinolaryngol 2019;276:3035-3041. https://doi.org/10.1007/s00405-019-05592-5
- Sproat R, Yiannakis C, Iyer A. Endoscopic stapes surgery: a comparison with microscopic surgery. Otol Neurotol 2017;38:662-666. https://doi.org/10.1097/MAO.0000000000001371
- Molinari G, Reale M, Bonali M, et al. Taste impairment after endoscopic stapes surgery: do anatomic variability of chorda tympani and surgical technique matter? Post-operative dysgeusia after EStS. Eur Arch Otorhinolaryngol 2022;279:2269-2277. https://doi.org/10.1007/s00405-021-06908-0
- Hoskison EE, Harrop E, Jufas N, et al. Endoscopic stapedotomy: a systematic review. Otol Neurotol 2021;42:E1638-E1643. https://doi.org/10.1097/MAO.0000000000003242
- Ito T, Kubota T, Furukawa T, et al. Measurement of the pediatric and adult osseous external auditory canal: implications for transcanal endoscopic ear surgery. Otol Neurotol 2020;41:E712-E719. https://doi.org/10.1097/MAO.0000000000002653
- Morizono T. Endaural approaches. Op Tech Otolaryngol Head Neck Surg 1996;7:66-70.
- Vital V, Konstantinidis I, Vital I, et al. Minimizing the dead ear in otosclerosis surgery. Auris Nasus Larynx 2008;35:475-479. https://doi.org/10.1016/j.anl.2007.11.002
- Wegner I, van Waes AMA, Bittermann AJ, et al. A systematic review of the diagnostic value of CT imaging in diagnosing otosclerosis. Otol Neurotol 2016;37:9-15. https://doi.org/10.1097/MAO.0000000000000924
- Brackmann DE, Shelton C, Arriaga MA, et al. Otologic surgery. Philadelphia: Elsevier, Inc; 2021.
- Balkany TJ, Telischi FF, Angeli SI, et al. Tympanomeatal flaps. Laryngoscope 2003;113:1266-1268. https://doi.org/10.1097/00005537-200307000-00028
- Fiorino F, Barbieri F. Reversal of the steps stapedotomy technique with early removal of the posterior crus: early postoperative results: how we do it. Clin Otolaryngol 2008;33:359-362. https://doi.org/10.1111/j.1749-4486.2008.01707.x
- Doménech J, Carulla M, Traserra J. Sensorineural high-frequency hearing loss after drill-generated acoustic trauma in tympanoplasty. Arch Otorhinolaryngol 1989;246:280-282. https://doi.org/10.1007/BF00463575
- Yavuz H, Caylakli F, Ozer F, et al. Reliability of microdrill stapedotomy: comparison with pick stapedotomy. Otol Neurotol 2007;28:998-1001. https://doi.org/10.1097/MAO.0b013e31815a3548
- Canale A, Albera A, Macocco F, et al. Microdrill stapedotomy for otosclerosis with small and large preoperative air-bone gap: a retrospective comparison of results. Acta Otolaryngol 2020;140:745-748. https://doi.org/10.1080/00016489.2020.1764618
- Mangham CA. Reducing footplate complications in small fenestra microdrill stapedotomy. Am J Otol 1993;14:118-121.
- Gjuric M. Microdrill versus perforator for stapedotomy. Clin Otolaryngol Allied Sci 1990;15:411-413. https://doi.org/10.1111/j.1365-2273.1990.tb00492.x
- Plodpai Y, Atchariyasathian V, Khaimook W. Endoscope-assisted stapedotomy with microdrill: comparison with a conventional technique. J Med Assoc Thai 2017;100:190-196.
- Palva T. Argon laser in otosclerosis surgery. Acta Otolaryngol 1987;104:153-157. https://doi.org/10.3109/00016488709109061
- Perkins RC. Laser stepedotomy for otosclerosis. Laryngoscope 1980;90:228-240. https://doi.org/10.1288/00005537-198002000-00007
- Kamalski DMA, Wegner I, Tange RA, et al. Outcomes of different laser types in laser-assisted stapedotomy: a systematic review. Otol Neurotol 2014;35:1046-1051. https://doi.org/10.1097/MAO.0000000000000270
- Silverstein H, Rosenberg S, Jones R. Small fenestra stapedotomies with and without KTP laser: a comparison. Laryngoscope 1989;99:485-488. https://doi.org/10.1288/00005537-198905000-00003
- Arnoldner C, Schwab B, Lenarz T. Clinical results after stapedotomy: a comparison between the erbium:yttrium-aluminum-garnet laser and the conventional technique. Otol Neurotol 2006;27:458-465. https://doi.org/10.1097/01.mao.0000217355.96334.ba
- Pauli N, Strömbäck K, Lundman L, et al. Surgical technique in stapedotomy hearing outcome and complications. Laryngoscope 2020;130:790-796. https://doi.org/10.1002/lary.28072
- Altamami NM, Huyghues des Etages G, Fleux M, et al. Is one of these two techniques: CO2 laser versus microdrill assisted stapedotomy results in better post-operative hearing outcome? Eur Arch Otorhinolaryngol 2019;276:1907-1913. https://doi.org/10.1007/s00405-019-05415-7
- Wegner I, Kamalski DMA, Tange RA, et al. Laser versus conventional fenestration in stapedotomy for otosclerosis: a systematic review. Laryngoscope 2014;124:1687-1693. https://doi.org/10.1002/lary.24514
- Hüttenbrink K-B. Biomechanics of stapesplasty: a review. Otol Neurotol 2003;24:548-557. https://doi.org/10.1097/00129492-200307000-00004
- Dancer A, Franke R. Biomechanics of the middle ear. Rev Laryngol Otol Rhinol (Bord) 1995;116:5-12.
- Grolman W, Tange RA, de Bruijn AJ, et al. A retrospective study of the hearing results obtained after stapedotomy by the implantation of two Teflon pistons with a different diameter. Eur Arch Otorhinolaryngol 1997;254:422-424. https://doi.org/10.1007/BF02439972
- Shabana YK, Ghonim MR, Pedersen CB. Stapedotomy: does prosthesis diameter affect outcome? Clin Otolaryngol Allied Sci 1999;24:91-94. https://doi.org/10.1046/j.1365-2273.1999.00207.x
- Colletti V, Fiorino FG. Stapedotomy with stapedius tendon preservation: technique and long-term results. Otolaryngol Head Neck Surg 1994;111:181-188. https://doi.org/10.1177/01945998941113P104
- Fucci MJ, Lippy WH, Schuring AG, et al. Prosthesis size in stapedectomy. Otolaryngol Head Neck Surg 1998;118:1-5. https://doi.org/10.1016/S0194-5998(98)70366-3
- Rosowski JJ, Merchant SN. Mechanical and acoustic analysis of middle ear reconstruction. Am J Otol 1995;16:486-497.
- de Bruijn AJ, Tange RA, Dreschler WA. Comparison of stapes prostheses: a retrospective analysis of individual audiometric results obtained after stapedotomy by implantation of a gold and a teflon piston. Am J Otol 1999;20:573-580.
- Robinson M. Stapes prosthesis: stainless steel vs. teflon. Laryngoscope 1974;84:1982-1995. https://doi.org/10.1002/lary.5540841114
- Cottle RD, Tonndorf J. Mechanical aspects of stapedial substitution. An experimental study. Arch Otolaryngol 1966;83:547-553. https://doi.org/10.1001/archotol.1966.00760020549010
- Gan RZ, Dyer RK, Wood MW, et al. Mass loading on the ossicles and middle ear function. Ann Otol Rhinol Laryngol 2001;110:478-485. https://doi.org/10.1177/000348940111000515
- Nishihara S, Goode RL. Experimental study of the acoustic properties of incus replacement prostheses in a human temporal bone model. Am J Otol 1994;15:485-494.
- Knox GW, Reitan H. Shape-memory stapes prosthesis for otosclerosis surgery. Laryngoscope 2005;115:1340-1346. https://doi.org/10.1097/01.mlg.0000172274.73365.11
- Fritsch MH, Naumann IC. Phylogeny of the stapes prosthesis. Otol Neurotol 2008;29:407-415. https://doi.org/10.1097/MAO.0b013e3181690775
- Roosli C, Schmid P, Huber AM. Biocompatibility of nitinol stapes prosthesis. Otol Neurotol 2011;32:265-270. https://doi.org/10.1097/MAO.0b013e318201622e
- Teschner M, Lilli G, Lenarz T. Comparison of superelastic nitinol stapes prostheses and platin teflon stapes prostheses. Eur Arch Otorhinolaryngol 2019;276:2405-2409. https://doi.org/10.1007/s00405-019-05476-8
- Ruckenstein MJ, Nicolli EA. Is there a ‘best’ stapes prosthesis? Laryngoscope 2012;122:2123-2124. https://doi.org/10.1002/lary.23353
- Silva VAR, Pauna HF, Lavinsky J, et al. Brazilian Society of Otology task force – Otosclerosis: evaluation and treatment. Braz J Otorhinolaryngol 2023;89:101303. https://doi.org/10.1016/j.bjorl.2023.101303
- Fisch U. Tympanoplasty, mastoidectomy, and stapes surgery. Stuttgart: Thieme; 2008.
- Huettenbrink K-B, Beutner D. A new crimping device for stapedectomy prostheses. Laryngoscope 2005;115:2065-2067. https://doi.org/10.1097/01.mlg.0000177988.05547.aa
- Bast F, Schrom T. First experiences with the new soft-clip piston as an alloplastic prosthesis during stapedotomy. Laryngorhinootologie 2009;88:304-308. https://doi.org/10.1055/s-0028-1100384
- Wegner I, Swartz JE, Bance ML, et al. A systematic review of the effect of different crimping techniques in stapes surgery for otosclerosis. Laryngoscope 2016;126:1207-1217. https://doi.org/10.1002/lary.25586
- Hornung JA, Brase C, Zenk J, et al. Results obtained with a new superelastic nitinol stapes prosthesis in stapes surgery. Otol Neurotol 2011;32:1415-1421. https://doi.org/10.1097/MAO.0b013e3182355886
- Schrötzlmair F, Surchan F, Pongratz T, et al. Laser-assisted fixation of a nitinol stapes prosthesis. Lasers Surg Med 2018;50:153-157. https://doi.org/10.1002/lsm.22738
- Fayad JN, Semaan MT, Meier JC, et al. Hearing results using the SMart piston prosthesis. Otol Neurotol 2009;30:1122-1127. https://doi.org/10.1097/MAO.0b013e3181be645d
- Shiao A-S, Kuo C-L, Cheng H-L, et al. Controversial issues of optimal surgical timing and patient selection in the treatment planning of otosclerosis. Eur Arch Otorhinolaryngol 2014;271:1007-1014. https://doi.org/10.1007/s00405-013-2529-6
- Rajan GP, Atlas MD, Subramaniam K, et al. Eliminating the limitations of manual crimping in stapes surgery? A preliminary trial with the shape memory Nitinol stapes piston. Laryngoscope 2005;115:366-369. https://doi.org/10.1097/01.mlg.0000154747.63561.52
- Tange RA, Grolman W. An analysis of the air-bone gap closure obtained by a crimping and a non-crimping titanium stapes prosthesis in otosclerosis. Auris Nasus Larynx 2008;35:181-184. https://doi.org/10.1016/j.anl.2007.04.007
- Scarpa A, Marra P, Ralli M, et al. Comparison of different oval window sealing materials in stapes surgery: systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2022;279:5521-5533. https://doi.org/10.1007/s00405-022-07551-z
- House JW. Stapedectomy technique. Otolaryngol Clin North Am 1993;26:389-393.
- Berrettini S, Burdo S, Forli F, et al. Far advanced otosclerosis: stapes surgery or cochlear implantation? J Otolaryngol 2004;33:165-171. https://doi.org/10.2310/7070.2004.03006
- Yellon RF, Thottam PJ. When should stapes surgery be performed in children? Laryngoscope 2015;125:2631-2632. https://doi.org/10.1002/lary.25235
- Coelho DH, Lee S, Yang E, et al. Subjective and objective taste change after stapes surgery systematic review and meta-analysis. Otol Neurotol 2023;44:10-15. https://doi.org/10.1097/MAO.0000000000003750
- Thiringer JK, Arriaga MA. Stapedectomy in military aircrew. Otolaryngol Head Neck Surg 1998;118:9-14. https://doi.org/10.1016/S0194-5998(98)70368-7
- Katzav J, Lippy WH, Shamiss A, et al. Stapedectomy in combat pilots. Am J Otol 1996;17:847-849.
- Hüttenbrink K-B. Clinical significance of stapedioplasty biomechanics: swimming, diving, flying after stapes surgery. Adv Otorhinolaryngol 2007;65:146-149. https://doi.org/10.1159/000098791
- Strutz J. Stapes-plasty and diving sports. Is diving after stapes-plasty really indicated?. HNO 1995;43:465, author reply 466.
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1654 times
- PDF downloaded - 150 times



