Reviews
Vol. 45: Issue 3 (Suppl. 1) - June 2025
Issues in the audiological assessment of otosclerosis
Abstract
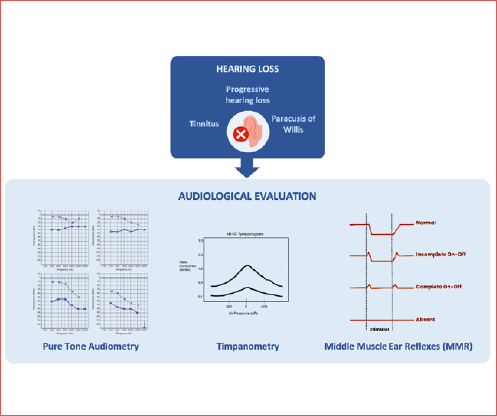
Otosclerosis is a temporal bone disease characterised by abnormal bone remodelling of the otic capsule. Typically, it presents as progressive conductive hearing loss, while sensorineural hearing loss may develop as the disease progresses. Diagnosis is challenging as no single diagnostic tool provides high specificity and sensitivity for otosclerosis. Instead, a battery of tests is used as proper diagnostic workup: pure-tone audiometry, tympanometry, and acoustic reflex tests are fundamental, while imaging, particularly high-resolution temporal bone computed tomography, enhances diagnostic specificity. Differential diagnosis should exclude middle ear pathologies, ossicular chain anomalies, osseous dystrophies, and inner ear disease such as third window disorders. As a result, a comprehensive, integrated diagnostic approach combining audiologic and imaging techniques is essential for accurate diagnosis and effective planning of treatment in otosclerosis.
Introduction
The term otosclerosis was coined by Politzer to describe a primary disease of the labyrinthine capsule, which was originally believed to be a condition attributed to “chronic interstitial middle ear catarrh” with secondary stapes fixation 1. Despite his publication of histologic evidence of otosclerosis in 16 cases of stapes fixation, it took almost half a century for Politzer’s views to gain universal acceptance 2.
Otosclerosis, a process of progressive pathologic bone remodelling affecting only the temporal bone, is one of the most complex diseases that leads to hearing loss (HL). It is characterised by abnormal remodelling in the otic capsule, particularly the fissula ante fenestram, but may extend to the region of the labyrinth and cochlea, oval, and round window 3,4.
The classic presentation of otosclerosis consists of progressive conductive HL (CHL) in adulthood, which is worse at low frequencies especially early in the disease. This is explained by the effect of the impaired vibrancy of the footplate in the lower frequencies. It occurs bilaterally in 80% of patients, although unilateral involvement is often present early in the disease.
The progression of otosclerosis should be monitored by an audiogram because it directly correlates to HL. As the stapes footplate becomes fixed to the oval window, the CHL worsens, and the air bone gap (ABG) increases and begins to involve all frequencies.
However, the type of deafness depends on the location and extension of the otosclerotic foci: lesions that originate in the fissula ante fenestram and involve the annular ligament cause conductive deafness, whereas medial progression to the cochlear endosteum causes sensorineural deafness. Therefore, when the course of otosclerosis deviates from the classic presentation, especially in the retrofenestral subtypes of the disease, mixed HL (MHL) or only sensorineural HL (SNHL) might occur 5,6.
Tinnitus is a highly prevalent symptom, and patients may describe improved hearing clarity in noisy environments. This phenomenon is known as paracusis of Willis, in which the CHL subdues the background noise such that it improves the signal-to-noise ratio for the patient.
Vestibular symptoms have been reported in up to 40% of patients with otosclerosis. They should be accurately investigated during clinical evaluation, since they can conceal pathologies mimicking otosclerosis, such as Ménière’s disease, an enlarged vestibular aqueduct (EVA), or superior semicircular canal dehiscence (SSCCD) 4.
A correct assessment of the disease is of utmost importance as it has the potential to lead to earlier diagnosis, referral, and treatment. Precise and accurate audiologic evaluation is fundamental to a proper surgical approach: otologists rely on the precision of the audiologic results and determination of the degree of the conductive component. Therefore, a precise audiologic work-up is a crucial part of the diagnostic protocol for otosclerosis (Cover figure).
Audiological diagnosis of otosclerosis
It must be clarified that there are currently no preoperative diagnostic evaluations specifically tailored for otosclerosis that can achieve sufficiently high levels of specificity or sensitivity for the disease. Nonetheless, combining multiple examinations, as a test battery, may aid in differential diagnosis and can generate a considerable clinical suspicion for otosclerosis.
Since the advent of modern stapedectomy pioneered by Shea in the 1950s, there have been significant advancements in diagnostic testing and a deeper comprehension of how concurrent otologic conditions can influence surgical outcomes. Audiological characteristics play a crucial role in diagnosing clinical otosclerosis. Generally, pure-tone audiometry, tympanometry, and acoustic reflex tests serve as diagnostic tools. The clinical diagnosis of stapes fixation hinges on various factors such as case history (progressive HL, tinnitus, paracusis, familial history, etc.), normal otoscopic findings, CHL and/or MHL, type A or As tympanograms, and the absence of the middle ear muscle reflex (MMR).
However, the combination of normal otoscopy with CHL is not exclusive to otosclerosis, with similar presentations being observed in cases of middle and inner ear anomalies. Further, a CHL with normal tympanogram and absent MMRs may be present in some middle ear or ossicular chain diseases. As a consequence, comprehensive audiological preoperative assessment is crucial for a correct approach.
Pure tone audiometry
In otosclerosis, stapes fixation and resultant stiffening of the ossicular chain almost always produces HL, particularly for lower frequency sounds. Typical findings from pure tone audiometry reveal a CHL, where air conduction sensitivity is notably poorer compared to bone conduction. A typical audiological sign of otosclerosis is the “Carhart notch”, a lowering of the bone hearing level up to 25 dB at 2 kHz; it has historically been considered an indicator of otosclerosis, but it is not pathognomonic of the disease 7. This artificial sensorineural component is caused by the missing resonance of the middle ear whose maximum is located by 2 kHz. Therefore the “Carhart notch” disappears postoperatively 8. Recent investigations have raised 3 general inquiries regarding the diagnostic significance and specificity of notching deficits in bone conduction thresholds at 2000 Hz. Firstly, reductions in bone conduction thresholds are often observed at other frequencies in otosclerosis patients. Additionally, the presence of a Carhart notch at 2000 Hz is not consistently observed in all cases of otosclerosis or ossicular chain fixation. Lastly, concerning this second point, patients with causes of CHL distinct from otosclerosis may exhibit a notch in bone conduction thresholds at 2000 Hz 9.
In advanced stages of otosclerosis, CHL progresses to MHL. Furthermore, in cases with cochlear involvement, moderate to profound SNHLs are encountered.
To determine the presence of ABG and its entity holds significant importance, mainly for surgical indications. Particularly notable is its gradual decline towards higher frequencies, which is indicative of the elastic rigidity of the ossicular chain, a crucial factor in stapes ankylosis.
The tuning fork tests may be useful to confirm the presence of an ABG. They are based on the same physical principles as the audiometric measurements and are very simple and quick to execute. Among tuning fork tests, Weber’s and Rinne’s tests are the most widely used. Weber’s test lateralises to the affected ear, whereas Rinne’s test is negative in the affected ear in the case of a CHL 9,10.
Another procedure to be mentioned is the sensorineural acuity level (SAL) test. The SAL test provides valuable clinical information for better definition of cochlear reserve and the entity of the ABG, particularly in cases in which it is difficult to precisely evaluate the bone conduction threshold. The SAL test plays a unique role in clinical audiology when performed with insert earphones and used as a supplement to conventional bone-conduction measurements to confirm ear-specific information on sensory hearing thresholds 9.
Impedance audiometry: tympanometry and middle ear muscle reflexes
Immittance measurement has been a longstanding tool in evaluating middle ear disorders. It comprises tympanometry and MMRs assessment. In individuals with otosclerosis, a common presentation includes type As (shallow type A tympanogram) or normal type A tympanograms, and absent MMRs.
At present, tympanometry is primarily conducted using a conventional low probe tone frequency. However, tympanometry carried out at this conventional low probe tone frequency (226 Hz) often fails to detect lesions that specifically impact the ossicular chain. For instance, the tympanogram yielded by a conventional 226 Hz tympanogram is typically insufficient to distinguish between a normal middle ear and ears affected by otosclerosis (stapes fixation). It has been shown that an abnormality is most clearly seen when the probe tone frequency approaches the resonant frequency. Middle ear pathologies, such as otosclerosis, affect the resonant frequency of the middle ear system, and in patients with otosclerosis it has been demonstrated a shift to higher frequencies of the resonant frequency of the middle ear. Therefore, using a probe tone near the resonant frequency may provide the most useful information regarding the differential diagnosis of middle ear pathologies. Several clinical and laboratory studies have reported prominent differences between healthy and otosclerotic ears when using higher probe tone frequencies 9.
In this regard, the introduction of multifrequency tympanometry (MFT) has facilitated tympanometry across a broad spectrum of probe tone frequencies. Recent research indicates that the identification of otosclerosis (or stapes fixation) is enhanced by employing measures derived from multifrequency tympanometry or by combining tympanometric variables in specific configurations 9,8.
Furthermore, several studies have indicated narrower tympanometry peaks, at traditional low tone probe frequency in ears affected by otosclerosis compared to healthy ears.
MMR assessment serves as a valuable adjunct to tympanometry for detecting the presence or absence of middle ear disorders, including otosclerosis. First described in humans by Lüscher in 1929, with practical testing outlined by Metz in 1946, MMR testing involves monitoring stapedius muscle contraction elicited by high-intensity auditory stimuli. The absence of MMR in the probe ear is attributed to the inability to monitor changes in immittance due to stiffening of the ossicular chain, preventing measurable changes in immittance resulting from stapedial muscle contraction.
In otosclerosis, the affected ear will lack reflexes with both ipsilateral and contralateral stimulations. Conversely, MMRs in the affected ear indicate a mobile stapes footplate and ossicular chain, allowing practitioners to screen for other potential causes of CHL. In cases of unilateral otosclerosis, MMR is typically absent when the stimulus and probe tone are presented to the affected side (ipsilateral mode). However, MMR may be elevated or absent in the contralateral mode, depending on the severity of the CHL. Finally, the contralateral MMR in the unaffected side is also absent when the probe is placed in the affected side 8,9.
In the early stages of otosclerosis, a biphasic reflex response (an on-off effect) has been observed, even before the onset of an ABG. This biphasic MMR response is characterised by a sudden increase in admittance upon switching the stimulus on and off, surrounding a central plateau at 0 11. An “incomplete” or “partial” on-off effect is characterised by the appearance of a more or less extensive negative deflection between the 2 positive deflections, simulating a normal reflex. This type of response is a transitional form between normal reflex and “complete” on-off effect and is closely related to the evolution of the oval window stapedial otosclerotic focus 11.
Finally, it must be mentioned that MMR can be normally present in otosclerosis if no focus affects the oval window 11.
Wideband acoustic immittance (WAI)
WAI represents a novel method for assessing the middle ear that allows researchers and clinicians to quantify the energy reflected or absorbed in the ear canal across a broad frequency range, typically spanning from 250 to 8000 Hz. Power absorbance (PA) is a ratio of absorbed power over the incident power and varies between 0 and 1. A value of 0 means all sound energy has been reflected and a value of 1 means all sound energy has been absorbed by the middle ear system. WAI offers several potential advantages over conventional tympanometry; notably, it measures across a wide frequency range (250-8000 Hz) and boasts rapid and easy testing, typically requiring only a few seconds to complete.
In comparison to conventional 226 Hz tympanometry, WAI exhibits significantly greater sensitivity to ossicular pathologies. Additionally, the patterns of absorbance vary depending on the condition of the middle ear, resulting in distinct absorbance patterns for different pathologies. Typically, a stiffening pathology leads to reduced absorbance over a specific frequency range. For instance, otosclerotic ears often display markedly increased reflectance between 400 and 1000 Hz. Shahnaz et al. 12 have determined that PA represents the most effective method for identifying ears with otosclerosis compared to 226 Hz and multifrequency tympanometry. In their study, PA successfully identified otosclerosis in 82% of the sample with a false positive rate of 17.2% 12. The research indicates that incorporating PA alongside other tools for assessing middle ear function will enhance the identification of otosclerotic ears in clinical practice 9,12.
Other diagnostic tests
Regarding speech perception, word recognition scores in quiet are good or excellent in most patients with otosclerosis, even in those with some apparent deficit in bone conduction hearing thresholds. While speech audiometry can offer valuable insights into speech comprehension, it is not obligatory for the preoperative assessment of otosclerosis. However, its execution is advisable in cases exhibiting a bone conduction threshold shift (MHL, SNHL) to either exclude or confirm the presence of the roll-over recruitment phenomenon. In selected cases it may be also helpful in the choice of the ear to be operated. Conversely, if hearing devices selection and fitting become necessary, speech audiometry becomes imperative 8.
Transient-evoked otoacoustic emission (TEOAE) or distortion product otoacoustic emission (DPOAE) measurements offer no relevant diagnostic assistance. Otoacoustic emissions (OAEs) cannot be recorded in individuals with middle ear dysfunction, including those with ossicular chain fixation and otosclerosis. Nevertheless, several recent publications have discussed a potential application for OAEs in assessing auditory function after “microtraumatic stapedotomy”. Despite variations in findings across studies and between patients, some reports suggest the appearance of detectable OAEs in the frequency range of 1000 to 1500 Hz, possibly linked to the normalisation of the middle ear’s resonance frequency following microtraumatic stapedotomy 8.
Study of vestibular evoked myogenic potentials (VEMPs) is useful in distinguishing otosclerosis from some inner ear pathologies that can cause a conductive gap, the most frequent being EVA and SSCCD, where VEMPs responses are present, generally with lower thresholds and higher amplitudes 13. The evaluation of VEMPs thresholds can be added in the diagnostic work-up of suspected otosclerosis in case of doubt, narrowing down the differential diagnosis in patients with “pseudo-conductive” components, and reducing costs by preventing unnecessary radiation exposure and unsuitable middle ear surgery 14. Even if this approach may avoid unnecessary middle ear surgery for ABGs with unknown causes, the exact diagnostic role of VEMPs testing in this field requires further systemic examinations 9,13, 14. Regarding the elicitation of VEMPs in patients with otosclerosis, it is generally reported that stapes fixations are associated with the absence of VEMPs. Anyway, the presence of VEMPs does not exclude otosclerosis, since despite the conductive deficit, VEMPs can be elicited in a small number of ears with otosclerosis. The air conduction-VEMPs are more vulnerable to CHL, whereas bone conduction-VEMPs are not; however, the low rate of bone conduction-VEMPs could be caused by inner ear damage 14.
Discussion
Otosclerosis, an osteodystrophy of the otic capsule, can pose a diagnostic challenge, and the most exact identification of otosclerosis is still based on postoperative histopathologic analysis of the removed ankylotic stapes footplates. Classically, diagnosis of otosclerosis has been based on medical history, physical examination, and audiologic testing; furthermore, in the last decades, the role of imaging has increased, leading to higher specificity in diagnosis of the disease. As of now, there is no definitive preoperative diagnostic protocol, nor has a consensus-based approach been established; this poses a critical question from a clinical perspective, due to numerous potential differential diagnoses. Conducting precise preoperative examinations becomes paramount to avoid resorting to “blind surgery,” such as explorative tympanotomy.
Around one-third of stapes fixation cases may not originate from otosclerosis, but from congenital or acquired ossicular chain anomalies. Furthermore, it is essential to differentiate other middle or inner ear disorders from stapes fixations to prevent unnecessary surgeries or potential complications during the procedure. Finally, otosclerosis must be distinguished from other osseous dystrophies, such as Paget’s disease and osteogenesis imperfecta. In Table I the main clinical pictures that must be differentiated from otosclerosis are reported.
Middle ear disorders, causing a CHL and from which otosclerosis should be differentiated, include serous otitis media, adhesive otitis media, tympanosclerosis, chronic otitis media, cholesteatoma, and middle ear tumours. These disorders are generally ruled out after accurate otomicroscopic examination and tympanometry 8.
Serous otitis media may complicate otosclerosis. The combination of serous otitis media and otosclerosis presents difficulties in differential diagnosis, which are usually resolved only after the healing of secretory otitis media.
In tympanosclerosis, the tympanogram is generally type B (flat tympanogram), due to increased stiffness of the tympanic membrane and/or ossicular chain, but it may also be biphasic, due to the coexistence of fibrous and/or calcific thickening areas and atrophy of the tympanic membrane. In tympanosclerosis limited to the ossicles and/or oval window and/or tympanic membrane, tympanometric curves of type Ad (abnormal increase in peak gradient) may be detected, due to partial or total atrophy of the tympanic membrane and interruption of the ossicular chain from erosion of the long process of the incus, or may also be type C or As, with normal otomicroscopy. In this latter case, the differential diagnosis with otosclerosis is intraoperative.
Congenital cholesteatoma, in its initial form with an intact tympanic membrane, can mimic the audiometry and tympanometry of otosclerosis. Otomicroscopy may indicate the presence of cholesteatoma in the middle ear cavity, appearing as a translucent pearl close to the malleus. Differential diagnosis can only be made with a high-resolution temporal bone computed tomography (CT) scan. In some cases, congenital cholesteatoma can be an intraoperative finding.
Benign tumours of the middle ear such as facial nerve schwannomas in its second portion, ceruminous adenomas, tegmen tympani meningiomas, and glomus tumours in the initial phase of growth can mimic the clinical and audiologic presentation of otosclerosis. Differential diagnosis is performed using high-resolution CT, complemented in some cases (facial nerve schwannoma) with contrast-enhanced magnetic resonance imaging.
Ossicular chain congenital or acquired anomalies, may also mimic otosclerosis. The commonest congenital ossicular abnormalities include stapes fixation, followed by incudo-stapedial joint discontinuity. Other congenital ossicular abnormalities described in the literature include absence of stapes, stapes suprastructure, stapedius tendon, incus, and oval window, as well as stapes fixation to the promontory and Fallopian canal, or other various malformations. Differences in the ABG at low and high frequencies in pure-tone audiometry, impedance audiometry, and MMRs are useful for distinguishing otosclerosis from ossicular chain anomalies 15. In ossicular chain anomalies, the ABG is generally larger than in otosclerosis and affects both low and high frequencies. Regarding the Carhart notch, Kan et al. 15 recently reported that this may be present both in otosclerotic patients and in patients with incudo-stapedial disjunction, without a significant difference between groups. Similarly Kashio et al. 16 reported that the incidence and depth of the Carhart notch were not statistically different between their stapes fixation, incudo-stapedial joint detachment, and malleus or incus fixation groups. Wegner et al. 7 in a systematic review concluded that the Carhart notch was a useful hint for the presence of otosclerosis, but that it could not be used to confirm diagnosis.
The study of MMRs is useful in differentiating otosclerosis from ossicular chain anomalies. With ossicular etiologies of CHL, acoustic reflexes change as the disease progresses. In otosclerosis, the reflexes may be initially normal, but as stapes fixation progresses, reflex amplitudes will decrease, and thresholds will increase, until eventually the reflexes cannot be longer detected. Also, in ossicular chain anomalies the MMRs may be variably present. The assessment of the on-off effect may further improve the value of MMRs in differentiating among aetiologies of CHL 17. However, combinations of several examinations including CT are needed for the differential diagnosis of ossicular chain diseases: subtle findings on high resolution CT of the temporal bone, such as the absence of the stapedius tendon and pyramidal process, or the small size of the oval widow, may alert the clinician to these rare malformations 7.
The aplasia of the anular ligament as a minor middle ear malformation comes along with the fixation of a stapes footplate. This diagnosis should be considered in young patients with clinical symptoms of otosclerosis. In these cases, stapedoplasty is also the preferred therapy 11.
In the case of traumatic interruption of the ossicular chain there may be a dislocation and detachment of the incus from the malleus or the fracture of the crura of the stapes. The audiologic differential diagnosis with otosclerosis is based solely on tympanometry indicating a type Ad tympanogram (abnormal compliance of the ossicular-tympanic system). In incus dislocation, the stapedial reflex is absent because the interruption of the ossicular chain is located upstream of the insertion site of the stapedius muscle. If the interruption is downstream, as in the traumatic fracture of the crura of the stapes, the stapedial reflex is normally present.
The majority of patients with significant CHL, with otherwise normal otoscopic examination results, will have an ossicular aetiology of HL. However, there have been reports of patients undergoing middle ear exploration with the intraoperative finding of a normal ossicular chain, despite a significant CHL. For many years, these patients were considered as having an ‘‘inner ear CHL’’. More recently, it has been found that third window disorders, such as SSCCD and EVA, can also cause CHL, and many patients with previously unclear causes of CHL have been found to have such a third window disorder 18. Third window lesions classically present with auditory (HL) and vestibular (Tullio and Hennebert syndromes) symptoms and can be suspected if an ABG is accompanied by normal tympanometry and present MMRs, or if an ABG is accompanied by absent MMRs associated with other findings not typical of otosclerosis (e.g. vertigo or autophony) 17. The diagnosis of third window defects is radiological: on temporal bone CT, specific anatomic defects include SSCCD, posterior semicircular canal dehiscence, perilabyrinthine fistula, EVA, X-linked stapes gusher, and bone dyscrasias 17-19.
Patients with SSCCD typically present with vertigo and nystagmus induced by loud noises (Tullio phenomenon) or increases in external auditory canal pressure (Hennebert sign) 19. At audiometry, a characteristic ABG results from increased bone and decreased air conduction 17,18,20. This phenomenon occurs most significantly at lower sound frequencies (below 1 kHz), a range at which acoustic energy is readily dissipated. At higher frequencies, there is a small or no gap because proportionally less acoustic energy is shunted by the third window. VEMPs testing may show abnormally low response thresholds on the side of pathology. The effective impedance is reduced; this reduction results in increased transmission of acoustic energy to the saccule 20.
A very rare condition is posterior semicircular canal dehiscence; this can occur sporadically or in association with superior canal dehiscence. Clinically, patients may also demonstrate the Tullio and Hennebert signs, while audiometry reveals an ABG at frequencies below 1 kHz 20.
Perilabyrinthine fistula may be caused by destructive middle ear processes that erode the attenuated otic capsule, producing communication with the inner ear. When this involves the semicircular canals, vestibule, and/or scala vestibuli side of the cochlea, third window mechanics can result. The most common aetiology is chronic middle ear inflammation, such as cholesteatoma or otitis media, and the lateral semicircular canal is most frequently involved, due to its location directly adjacent to the middle ear. On audiometry, cholesteatoma demonstrates a characteristic ABG of middle ear origin, which is present at both low and high sound frequencies due to superimposed ossicular chain pathology. Rarely, cochlear-carotid dehiscence with the absence of the intervening bony partition can also occur. On audiometry, this condition demonstrates an ABG that is greater at lower frequencies, similar to other third window lesions. Other potential causes of perilabyrinthine fistula include trauma, surgery, and benign and neoplastic masses 20.
Among third window defects, much attention has to be paid to EVA, as it represents the most frequent inner ear malformation. It can present in adulthood as a mixed HL mimicking otosclerosis, from which it can be difficult to distinguish without temporal bone CT scan. At audiometry, patients present with a complex and variable pattern of HL. The sensorineural component of HL is thought to result from potential associated cochleovestibular malformations and manifests at higher sound frequencies. The CHL results from acoustic energy dissipation through an enlarged third window where the vestibular aqueduct joins the vestibule 18. This is shown by an audiometric ABG at low frequencies but may be missed if bone conduction is not measured, particularly in young children who do not tolerate a full audiologic examination 20. Wieczorek et al. 21 in a retrospective study underlined that an unusual history of HL, particularly when dating to childhood or MHL in patient populations less prone to otosclerosis, can raise the index of suspicion for adult EVA.
Interestingly, in 2016 Hong et al. 17 reported about the possibility of a concomitant otosclerosis and third window defect in patients with significant CHL. In their paper, the authors reported on 5 ears with concurrent radiological evidence of otosclerosis and SSCCD 17. When one considers middle ear exploration for concurrent otosclerosis and third window disorders, two issues have to be considered: if hearing can be improved significantly with middle ear surgery, given the concurrent presence of a third window disorder, and whether there is an increased risk of SNHL following stapedectomy, because of a concurrent third window disorder. The literature is sparce on this topic. Therefore, it seems reasonable to offer middle ear exploration to patients with concurrent otosclerosis and third window disorders, as long as they are counselled that there may not be complete closure of the ABG after surgery, and that especially in cases with EVA and X-linked third window a sensorineural further deficit may occur 17.
An extremely rare entity that may be a confounding factor in the diagnosis of otosclerosis is the presence of a “cochlear cleft” 22. It may mimic an otosclerotic focus on CT, and should be considered in the differential diagnosis when audiometric findings do not agree or when other radiological manifestations potentially indicate a CHL of inner ear origin. The ‘‘cochlear cleft’’ has been described radiographically as a curvilinear area of pericochlear hypodensity just anterior to the oval window on CT. It has been hypothesised to be related to the fissula ante fenestram, cartilaginous masses, incomplete ossification, a cleft between endosteal and periosteal bone, or related to the first ossification centre. Further studies have assessed the prevalence of the cochlear cleft in children and have shown no correlation with otologic symptoms. It is found less often with advancing age 22,23. The importance of distinguishing these lesions from an otosclerotic focus lies in the fact that they preclude stapedotomy. No improvement in hearing would occur, and surgery is thus unnecessary.
Regarding osteodystrophies of the otic capsule, Paget’s disease and osteogenesis imperfecta may present a clinical picture mimicking otosclerosis. Patients with osteogenesis imperfecta sometimes first present with a progressive CHL caused by bone reconstruction and consecutive fixation of the footplate. The therapy is stapedoplasty in accordance with the otosclerosis 8. Osteogenesis imperfecta is a congenital hereditary disorder characterised by abnormalities in collagen tissue. This condition can cause a stapedio-oval joint ankylosis that closely resembles otosclerosis. HL in osteogenesis imperfecta typically occurs in the second to third decade of life and can be conductive, mixed, or sensorineural. SNHL results from microfractures, haemorrhages, and the presence of vascular and fibrous repair tissue within and around the cochlea. Surgical intervention for stapedio-oval joint ankylosis in osteogenesis imperfecta is indicated, although the results are generally modest. Surgical management of HL in osteogenesis imperfecta poses technical challenges because the stapes footplate is fragile and covered by a thick, highly vascularised mucoperiosteum.
Cochlear otosclerosis
A rare and controversial entity that deserves mention is cochlear otosclerosis. It is defined as a focus of otosclerosis located in the otic capsule involving the cochlear endosteum and causing SNHL without any stapes fixation or any conductive component. However, Schucknecht et al. 24,25. clearly showed that, when otosclerosis is sufficiently severe to involve the cochlear endosteum, it usually fixes the stapes as well. If the definition of cochlear otosclerosis is accepted as the involvement of cochlear endosteum without associated stapes fixation, then the incidence among ears with pure progressive SNHL is very rare, reportedly 0.3-1% 24,26.
In the clinical diagnosis of cochlear otosclerosis, some criteria are suggested in the literature, such as SNHL with good speech discrimination, a family history of otosclerosis in a patient with symmetrical SNHL, and a positive Schwartze’s sign 27. SNHL in cochlear otosclerosis may be suddenly aggravated at puberty or at periods of endocrine activity such as pregnancy, menopause, or treatment with oestrogens 26.
The diagnosis of cochlear otosclerosis mainly depends on temporal bone high-resolution CT 24,26. The fact that there are some clinical studies with imaging techniques which report patients with a pure cochlear sensorineural type of otosclerosis, while otopathologic studies indicate that this type of ear pathology is very rare, points to the need for more clinical control studies in large series.
Juvenile otosclerosis
Even if considered an adult disease, otosclerosis can appear during childhood as well 28. It is estimated that approximately 15% of patients with otosclerosis experienced HL before the age of 18 years 28. A histologic study reported an incidence of less than 1% in children aged under 5 years and 4% in children aged between 5 and 18 years 28, indicating that clinical expression of the disease is even lower. The main revealing feature of juvenile otosclerosis (JO) is CHL, and therefore the precision in establishing ABG, based on masking techniques and immittance evaluations, have a key role in the diagnosis. Pure tone audiometry, tympanometry, and acoustic reflexes must be combined into a test battery 28, especially in children as the typical CHL feature (a distinct notch-like decrease in bone conduction thresholds around 2000 Hz) is not always identified 28. CT is a necessary step for diagnosis and, in recent years, high-resolution cone beam CT has been used to assess middle and inner ear with a significantly lower effective radiation dose than traditional CT 28,29.
The rare cases of JO must be differentiated from other diseases causing CHL in children to plan proper treatment. Serous otitis media is the most frequent cause of CHL in childhood, and may be a confounding factor when coexisting with JO. To this regard, in 2016 Markou et al. 29 reported on a case with coexisting otosclerosis and serous otitis media, presenting with persistent CHL after resolution of the otitis.
Besides JO, other causes of stapes footplate ankylosis are congenital stapes footplate fixation (CSFF), Paget’s disease, and osteogenesis imperfecta. CSFF is often non-progressive, with no clear family history, and is generally recognised at a younger age compared to JO 30. It can be seen as an isolated anomaly or together with other ossicular chain abnormalities 30. It has been associated with a greater ABG compared to JO and with poorer postoperative hearing outcomes 30. It is often difficult to preoperatively distinguish between the two disease entities given the similarities in presentation 30.
Conclusions
A diagnosis of otosclerosis can usually be easily established through a comprehensive clinical approach, combining medical history, pure tone audiometry, impedance audiometry, and assessment of MMRs. However, it can sometimes pose diagnostic challenges, so that an integrated diagnostic approach is essential, especially for dealing with ambiguous cases. This approach not only requires extensive audiologic examination, but also, in selected cases, the use of imaging techniques, particularly high-resolution CT, for accurate differential diagnosis and to plan appropriate treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
FF, SC, ADV: conceived the initial idea for the narrative review and developed the structure of the article; LB, FL: performed the literature review and critically analyzed the sources. All authors contributed significantly to drafting the manuscript, revising it for important intellectual content, and approving the final version to be submitted. Each author has read and agreed to the published version of the manuscript.
Ethical consideration
Not applicable.
History
Received: October 1, 2024
Accepted: April 7, 2025
Figures and tables
| Middle ear pathologies | Serous otitis media |
| Adhesive otitis media and tympanosclerosis | |
| Chronic otitis media and cholesteatoma | |
| Middle ear tumours | |
| Ossicular chain anomalies | Congenital and acquired ossicular chain anomalies |
| Inner ear anomalies (third window disorders) | Superior semicircular canal dehiscence |
| Posterior semicircular canal dehiscence | |
| Perilabyrinthine fistula | |
| Enlarged vestibular aqueduct | |
| X-linked stapes gusher | |
| Osseous dystrophies | Paget’s disease |
| Osteogenesis imperfecta | |
| Others | Cochlear cleft |
| Juvenile otosclerosis | |
| Cochlear otosclerosis |
References
- Politzer A. Über primäre erkrankung der knöchernen labyrinthkapsel. Zeitschr Ohrenheil. 1893;25:309-327.
- McElveen J, Kutz J. Controversies in the evaluation and management of otosclerosis. Otolaryngol Clin North Am. 2018;51:487-499. doi:https://doi.org/10.1016/j.otc.2017.11.017
- Batson L, Rizzolo D. Otosclerosis: an update on diagnosis and treatment. JAAPA. 2017;30:17-22. doi:https://doi.org/10.1097/01.JAA.0000511784.21936.1b
- Silva V, Pauna H, Lavinsky J. Brazilian Society of Otology task force – Otosclerosis: evaluation and treatment. Braz J Otorhinolaryngol. 2023;89. doi:https://doi.org/10.1016/j.bjorl.2023.101303
- Berrettini S, Lombardo F, Bruschini L. 3D fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging at different stages of otosclerosis. Eur Arch Otorhinolaryngol. 2018;275:2643-2652. doi:https://doi.org/10.1007/s00405-018-5093-2
- Lombardo F, De Cori S, Aghakhanyan G. 3D-Flair sequence at 3T in cochlear otosclerosis. Eur Radiol. 2016;26:3744-3751. doi:https://doi.org/10.1007/s00330-015-4170-9
- Wegner I, Bittermann A, Hentschel M. Pure-tone audiometry in otosclerosis: insufficient evidence for the diagnostic value of the Carhart notch. Otolaryngol Head Neck Surg. 2013;149:528-532. doi:https://doi.org/10.1177/0194599813495661
- Sziklai I, ed. Surgery of Stapes Fixations. Springer International Publishing; 2016. doi:https://doi.org/10.1007/978-3-319-28576-4
- Danesh A, Shahnaz N, Hall J. The audiology of otosclerosis. Otolaryngol Clin North Am. 2018;51:327-342. doi:https://doi.org/10.1016/j.otc.2017.11.007
- Probst R. Adv Otorhinolaryngol. (Arnold W, Häusler R, eds.).; 2007. doi:https://doi.org/10.1159/000098754
- Thomas J, Minovi A, Dazert S. Current aspects of etiology, diagnosis and therapy of otosclerosis. Otolaryngol Pol. 2011;65:162-170. doi:https://doi.org/10.1016/S0030-6657(11)70670-9
- Shahnaz N, Bork K, Polka L. Energy reflectance and tympanometry in normal and otosclerotic ears. Ear Hear. 2009;30:219-233. doi:https://doi.org/10.10977/AUD.0b013e3181976a14
- Zhou G, Poe D, Gopen Q. Clinical use of vestibular evoked myogenic potentials in the evaluation of patients with air-bone gaps. Otol Neurotol. 2012;33:1368-1374. doi:https://doi.org/10.1097/MAO.0b013e31826a542f
- Tramontani O, Gkoritsa E, Ferekidis E. Contribution of Vestibular-Evoked Myogenic Potential (VEMP) testing in the assessment and the differential diagnosis of otosclerosis. Med Sci Monit. 2014;20:205-213. doi:https://doi.org/10.12659/MSM.889753
- Kan T, Ueda H, Kishimoto M. Availability of audiological evaluation for the differential diagnosis of clinical otosclerosis. Auris Nasus Larynx. 2020;47:343-347. doi:https://doi.org/10.1016/j.anl.2020.03.009
- Kashio A, Ito K, Kakigi A. Carhart notch 2-kHz bone conduction threshold dip: a nondefinitive predictor of stapes fixation in conductive hearing loss with normal tympanic membrane. Arch Otolaryngol Head Neck Surg. 2011;137:236-240. doi:https://doi.org/10.1001/archoto.2011.14
- Hong R, Metz C, Bojrab D. Acoustic reflex screening of conductive hearing loss for third window disorders. Otolaryngol Head Neck Surg. 2016;154:343-348. doi:https://doi.org/10.1177/0194599815620162
- Merchant S, Rosowski J. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29:282-289. doi:https://doi.org/10.1097/mao.0b013e318161ab24
- Minor L, Solomon D, Zinreich J. Sound– and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124:249-258. doi:https://doi.org/10.1001/archotol.124.3.249
- Ho M, Moonis G, Halpin C. Spectrum of third window abnormalities: semicircular canal dehiscence and beyond. AJNR Am J Neuroradiol. 2017;38:2-9. doi:https://doi.org/10.3174/ajnr.A4922
- Wieczorek S, Anderson M, Harris D. Enlarged vestibular aqueduct syndrome mimicking otosclerosis in adults. Am J Otolaryngol. 2013;34:619-625. doi:https://doi.org/10.1016/j.amjoto.2013.07.015
- Van Rompaey V, Potvin J, Van Den Hauwe L. Third mobile window associated with suspected otosclerotic foci in two patients with an air-bone gap. J Laryngol Otol. 2011;125:89-92. doi:https://doi.org/10.1017/S0022215110001544
- Pucetaite M, Quesnel A, Juliano A. The cochlear cleft: CT correlation with histopathology. Otol Neurotol. 2020;41:745-749. doi:https://doi.org/10.1097/MAO.0000000000002637
- Lu S, Wei X, Chen B. A new phenomenon of cochlear otosclerosis: an acquired or congenital disease? – A clinical report of cochlear otosclerosis. Acta Otolaryngol. 2021;141:551-556. doi:https://doi.org/10.1080/00016489.2021.1906947
- Schuknecht H, Kirchner J. Cochlear otosclerosis: fact or fantasy. Laryngoscope. 1974;84:766-782. doi:https://doi.org/10.1288/00005537-197405000-00008
- Cureoglu S, Baylan M, Paparella M. Cochlear otosclerosis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:357-362. doi:https://doi.org/10.1097/MOO.0b013e32833d11d9
- Shambaugh G. Therapy of cochlear otosclerosis. Ann Otol Rhinol Laryngol. 1966;75:579-583. doi:https://doi.org/10.1177/000348946607500222
- Fancello V, Sacchetto L, Bianchini C. Management of juvenile otosclerosis: a systematic review. Children. 2022;9. doi:https://doi.org/10.3390/children9111787
- Markou K, Stavrakas M, Karkos P. Juvenile otosclerosis: a case presentation and review of the literature. BMJ Case Rep. Published online 2016. doi:https://doi.org/10.1136/bcr-2015-214232
- Daniel A, Budiono G, Rao A. Juvenile otosclerosis and congenital stapes footplate fixation. A systematic review and meta-analysis of surgical outcomes and management. Intern J Pediatr Otorhinolaryngol. 2023;166. doi:https://doi.org/10.1016/j.ijporl.2022.111418
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1579 times
- PDF downloaded - 233 times



