Reviews
Vol. 45: Issue 3 (Suppl. 1) - June 2025
Implantable hearing aids in otosclerosis: indications, surgical applications, and cost-effectiveness
Abstract
Since the 1980s, implantable hearing devices have revolutionised otologic surgery, providing new options for patients with otosclerosis. This article discusses two primary types of devices that are beneficial to patients with otosclerosis: bone anchored hearing devices (BAHDs) and active middle ear implants (AMEIs). BAHDs include percutaneous, passive, and active transcutaneous devices, offering an alternative for patients where stapes surgery or conventional hearing aids are not feasible. Although BAHDs improve audiological outcomes, they are generally considered a third-line treatment due to their limited cost-effectiveness ratio in otosclerosis. AMEIs, such as the Vibrant Soundbridge, are another option, offering superior speech recognition without the occlusion effects of traditional hearing aids. While implantable hearing devices show promising results, they are typically reserved for patients at high surgical risk or who do not benefit from conventional hearing aids. Further cost-benefit analysis is needed, as implantable devices are less economically favourable compared to stapes surgery.
Introduction
Since the mid-1980s 1, otologic surgery has been revolutionised by the advent of implantable prostheses, which have modernised the approach to hearing rehabilitation in patients with otologic issues.
Since then, numerous prostheses with different characteristics have been developed to address the hearing deficit of patients. Among these, only some are indicated and have been used in patients affected by otosclerosis:
- bone anchored hearing devices (BAHDs);
- active middle ear implants (AMEIs).
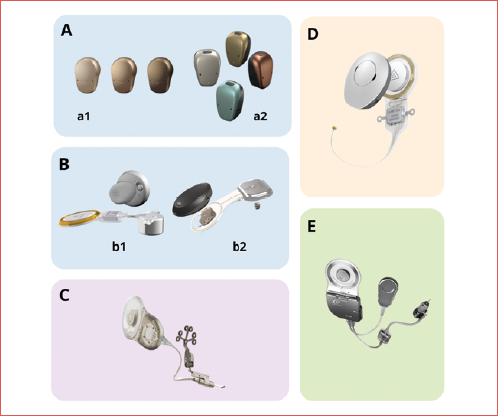
The aim of this paper is to clarify indications and surgical applications of implantable hearing aids in patients affected by otosclerosis (Cover figure).
Bone anchored hearing devices
The first to have been developed are bone-anchored prostheses. The use of penetrating implants was initially described in 1977 1, but it was a further 10 years before the devices became commercially available. At present, the BAHDs available are:
- percutaneous bone-conduction devices such as the Baha Connect (Cochlear BAS, Gothenburg, Sweden) and the Ponto System (Oticon Medical AB, Askim, Sweden). These involve a titanium screw implanted in the skull bone, protruding through the skin to which an external audio processor is attached;
- passive transcutaneous bone-conduction devices such as the Baha Attract (Cochlear BAS, Gothenburg, Sweden) and Sophono (Medtronic, Jacksonville, FL). These devices do not penetrate the skin but use magnetic coupling to transmit sound from processor to the bone where the magnet is attached. The sound vibration passes through the skin;
- active transcutaneous bone-conduction devices such as the Bonebridge (MED-EL, Innsbruck, Austria) and Osia 2 (Cochlear BAS, Gothenburg, Sweden), in which an active implant (with magnetic or piezoelectric transductor) is placed under the skin and muscles of the temporal region and communicates with the external sound processor wirelessly via radiofrequency.
These devices have been widely used in patients affected by otosclerosis 2,3; however, they are considered only as a third option in the treatment of hearing loss 2.
Studies have shown the benefits of BAHDs in terms of audiological performance and overall patient quality of life 4-6, even in patients affected by otosclerosis, although the benefits are slightly less pronounced compared to patients with other middle ear pathologies 3.
As most recent paper underlines 7,8, these devices are recommended only in cases of surgical failure, or in cases in which a surgical revision exposes the patient to a high risk of deafness and conventional hearing aids cannot be fitted. The Brazilian task force on otosclerosis 7 considers the main indications of BAHDs as:
- eczematous otitis externa precluding the use of conventional hearing aids or no adequate gain obtained with the device;
- unfavourable surgical anatomy (persistent stapedial artery, obliteration of the oval window by a dehiscent facial nerve);
- otosclerosis in single-sided deafness;
- revision surgery.
Some authors have also focused attention on the progressive pattern of otosclerosis and the risk of deterioration of cochlear reserve over the years, highlighting the possibility that BAHDs may no longer achieve the appropriate auditory performance 7.
In an evolving era of healthcare management, it is wise to reflect on the value of therapies to ensure high-quality care reaches all those who need it, avoiding useless treatments or those with an unfavourable cost-benefit ratio. In the literature, there are no studies analysing the issue of cost-effectiveness of BAHDs in the subgroup of patients with otosclerosis. However, there are studies 9 that have shown that stapes surgery is less expensive than treating patients with conventional hearing aids. Gillard et al. 10 assert that stapes surgery represents a beneficial and cost-effective strategy to treat otosclerosis, as it enhances quality of life while reducing expenses. Other authors 11 indicate that the cost-effectiveness of BAHDs compared to conventional hearing aid devices in all types of patients remains uncertain. Considering the high cost of the devices, the cost-effectiveness ratio is likely unfavourable in patients affected by otosclerosis when compared to the other 2 options (conventional hearing aids and stapes surgery). However, further studies on cost-benefit should be carried out to clarify the ratio between BAHDs, conventional hearing aid, and stapes surgery.
Active middle ear implants
AMEIs emerged in the 1990s as a treatment option for patients who are unable to use hearing aids. They offer functional gain with improved speech recognition that surpasses that of conventional hearing aids, and most do so without causing occlusion effect or feedback. There are two main groups of AMEIs: semi-implantable (siAMEI) or fully-implantable (fiAMEI).
In patients affected by otosclerosis, there are 3 implants that have been mostly used: the Vibrant Soundbridge (VSB, Med-El, Innsbruck, Austria) and Codacs (Cochlear Ltd., Sydney, Australia) in the siAMEI group, and Carina (Cochlear Ltd., Sydney, Australia) in the fiAMEI. Currently, the only AMEI still available is the Vibrant Soundbridge.
The VSB is made by 2 components: an external sound processor and an internal part called vibrating ossicular replacement prosthesis (VORP) 12,13. The external component consists of a microphone, audio processor, battery, transmitter, and magnet. It converts acoustic signals into an amplitude-modulated signal and transmits them via electromagnetic waves to the VORP 13. This consists of a receiver coil, conductor link, and floating mass transducer (FMT). The FMT, the crucial component of the VSB, contains an electromagnetic coil within a titanium housing that encloses a small magnet. When it is connected to a moving structure (ossicles, round or oval window), these vibrations can be transmitted to stimulate the fluids of the inner ear.
The VSB may be indicated in patients suffering from otosclerosis with stable hearing loss for at least 12 months. In patients with conductive hearing loss who do not adapt to or gain little benefit from conventional hearing aids and are unwilling to accept the risks of stapedotomy, the FMT can be placed on the short process of the incus using a titanium clip 7. For patients with moderate to severe mixed hearing loss, the VSB can also be placed during or after stapes surgery 7,8,14. This indication is the only one taken into consideration in an Italian consensus 8 even though this was strongly debated among the authors.
Codacs is an implantable hearing system, first reported by Hausler in 2008 15, which directly stimulates the inner ear by vibrating the perilymph. The Codacs system comprises an implantable part, consisting of a receiver coil, the implant electronics, and the electromagnetic actuator, which is held in place by a fixation system, and an externally behind-the-ear sound processor with a radio-frequency coil. The actuator has a terminal – referred to as the ‘artificial incus’ – which is similar in size to the long process of the incus. The artificial incus should be positioned in such a way that it is aligned to the level of the natural incus, above the oval window, while avoiding contact with the surrounding tissues and bony structures. After positioning the artificial incus by posterior tympanotomy 15 or superior-anterior tympanotomy 16, a traditional stapes prosthesis is inserted into the footplate perforation and secured to the long process of the artificial incus of the actuator by crimping. The actuator transforms electrical signal into mechanical vibrations and stimulate the cochlea by applying a vibration to the stapes prosthesis through the artificial incus bypassing the outer and middle ear. The indication range of the device is a bone conduction threshold between 40 and 80 dB (0.5-4 kHz) and an air conduction threshold higher than 60 dB. Results reported in the literature are good. Busch et al. 17, in a series of 10 patients, reported an average functional gain (0.5-4 kHz) achieved with conventional hearing aids of 47 dB, compared to 56 dB with Codacs. Hausler et al. 15 reported, in a case series of 4 patients, a decrease of air bone gap by 10-25 dB at pure tone audiometry.
Carina is a fully implantable hearing aid; it comprises a microphone, rechargeable battery, magnet, sound processor, actuator, and transducer. The transducer is positioned on the body of the incus. Furthermore, the Carina system features an external charger that utilises magnetic contact for device charging, along with a remote control to adjust volume and power settings. There are no visible external components, thereby resolving many of the problems (such as swimming, sports activities, and dusty work environments) related to the use of external acoustic processors. Carina is designed to address the amplification needs of adults, > 18 years of age, with moderate to severe sensorineural hearing loss and normal middle ears, providing a mechanical direct stimulation of the middle ear ossicles. However, over the years the development of extension of the actuator allowed to broaden the surgical indications. With these extensions the actuator could be positioned on middle ear: stapes, oval window, or round window. Literature reports on patients with otosclerosis undergoing Carina implantation are anecdotal 18-20 and all patients are implanted on oval window and following multiple surgeries for otosclerosis.
Conclusions
Currently, the only implantable hearing aids for patients with otosclerosis are bone-anchored hearing aids and the VSB. The use of these devices in patients affected by otosclerosis represents a third-line therapeutic option for those at high surgical risk for stapes surgery, and in cases in which there is no indication for conventional hearing aids. Implantable prostheses can achieve good auditory outcomes; however, due to their unfavourable cost-benefit ratio they should be reserved for highly selected cases.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
ADV, LB, FF: conceived the initial idea for the narrative review and developed the structure of the article; SB: performed the literature review and critically analyzed the sources. All authors contributed significantly to drafting the manuscript, revising it for important intellectual content, and approving the final version to be submitted. Each author has read and agreed to the published version of the manuscript.
Ethical consideration
Not applicable.
History
Received: January 27, 2025
Accepted: April 7, 2025
References
- Tjellström A, Lindström J, Hallén O. Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol. 1981;2:304-310.
- Burrell S, Cooper H, Proops D. The bone anchored hearing aid – The third option for otosclerosis. J Laryngol Otol (Suppl.). 1996;21:31-37.
- McLarnon C, Davison T, Johnson I. Bone-anchored hearing aid: comparison of benefit by patient subgroups. Laryngoscope. 2004;114:942-944. doi:https://doi.org/10.1097/00005537-200405000-00030
- Lustig L, Arts H, Brackmann D. Hearing rehabilitation using the BAHA bone-anchored hearing aid: results in 40 patients. Otol Neurotol. 2001;22:328-334. doi:https://doi.org/10.1097/00129492-200105000-00010
- Wazen J, Spitzer J, Ghossaini S. Results of the bone-anchored hearing aid in unilateral hearing loss. Laryngoscope. 2001;111:955-958. doi:https://doi.org/10.1097/00005537-200106000-00005
- Browning G, Gatehouse S. Estimation of the benefit of bone-anchored hearing aids. Ann Otol Rhinol Laryngol. 1994;103:872-878. doi:https://doi.org/10.1177/000348949410301108
- Silva V, Pauna H, Lavinsky J. Brazilian Society of Otology task force – Otosclerosis: evaluation and treatment. Braz J Otorhinolaryngol. 2023;89. doi:https://doi.org/10.1016/j.bjorl.2023.101303
- Bruschini L, Canzi P, Canale A. Implantable hearing devices in clinical practice. Systematic review and consensus statements. Acta Otorhinolaryngol Ital. 2024;44:52-67. doi:https://doi.org/10.14639/0392-100X-N2651
- Bonnafous S, Margier J, Bartier S. Estimated costs associated with management of otosclerosis with hearing aids vs surgery in Europe. JAMA Netw Open. 2022;5. doi:https://doi.org/10.1001/jamanetworkopen.2021.48932
- Gillard D, Harris J. Cost-effectiveness of stapedectomy vs hearing aids in the treatment of otosclerosis. JAMA Otolaryngol Head Neck Surg. 2020;146:42-48. doi:https://doi.org/10.1001/jamaoto.2019.3221
- Crowson M, Tucci D. Mini review of the cost-effectiveness of unilateral osseointegrated implants in adults: possibly cost-effective for the correct indication. Audiol Neurootol. 2016;21:69-71. doi:https://doi.org/10.1159/000443629
- Grégoire A, Van Damme J, Gilain C. Our auditory results using the Vibrant Soundbridge on the long process of the incus: 20 years of data. Auris Nasus Larynx. 2018;45:66-72. doi:https://doi.org/10.1016/j.anl.2017.02.007
- Bruchhage K, Leichtle A, Schönweiler R. Systematic review to evaluate the safety, efficacy and economical outcomes of the Vibrant Soundbridge for the treatment of sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2017;274:1797-1806. doi:https://doi.org/10.1007/s00405-016-4361-2
- Burian A, Gerlinger I, Toth T. Stapedotomy with incus vibroplasty – A novel surgical solution of advanced otosclerosis and its place among existing therapeutic modalities – Hungarian single institutional experiences. Auris Nasus Larynx. 2020;47:55-64. doi:https://doi.org/10.1016/j.anl.2019.04.004
- Häusler R, Stieger C, Bernhard H. A novel implantable hearing system with direct acoustic cochlear stimulation. Audiol Neurootol. 2008;13:247-256. doi:https://doi.org/10.1159/000115434
- Bruschini L, Forli F, De Vito A. A new surgical approach for direct acoustic cochlear implant: a temporal bone study. Clin Exp Otorhinolaryngol. 2016;9:314-318. doi:https://doi.org/10.21053/ceo.2015.01739
- Busch S, Kruck S, Spickers D. First clinical experiences with a direct acoustic cochlear stimulator in comparison to preoperative fitted conventional hearing aids. Otol Neurotol. 2013;34:1711-1718. doi:https://doi.org/10.1097/MAO.0000000000000225
- Bruschini L, Berrettini S, Forli F. The Carina© middle ear implant: surgical and functional outcomes. Eur Arch Otorhinolaryngol. 2016;273:3631-3640. doi:https://doi.org/10.1007/s00405-016-3998-1
- Didczuneit-Sandhop B, Langer J. Use of the Carina active middle ear implant in otosclerosis patients. HNO. 2021;69:828-834. doi:https://doi.org/10.1007/s00106-020-00984-0
- Martin C, Deveze A, Richard C. European results with totally implantable Carina placed on the round window: 2-year follow-up. Otol Neurotol. 2009;30:1196-1203. doi:https://doi.org/10.1097/MAO.0b013e3181c34898
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1035 times
- PDF downloaded - 220 times



