Rhinology
Vol. 45: Issue 3 - June 2025
Anatomical basis of the elongated nasoseptal flap
Abstract
Objective. The aim of this study was to elucidate the anatomical factors influencing elongation of the pedicle of the nasoseptal flap (NSF).
Methods. Dissections were conducted on tissue blocks taken from 61 embalmed half-heads. In the first group, 36 were microdissected to delineate the configuration of the third part of the maxillary artery (MA). In the second group, 25 other specimens were dissected using endoscopic instruments to determine the tethering points limiting the mobility of the NSF and to document the surgical manoeuvres required for release.
Results. The MA is coiled in the pterygopalatine fossa (PPF) into a variety of configurations that can be classified into single-looped (SL) or double-looped (DL) forms depending on the number of vessel loops. Up to 4.7 ± 0.5 cm of NSF pedicle elongation is possible in the more common (80.8%) DL forms. Elongation is limited to 2.6 ± 0.5 cm in the SL form; greater palatine artery transection is more important to pedicle elongation in the SL form. Posterior branches such as the pharyngeal and vidian arteries may hinder pedicle elongation.
Conclusions. The pedicle of the NSF may be elongated by up to 5 cm, greatly increasing its potential utility. The vessel length available for elongation is determined by the extent of MA looping, while the branch configuration, and particularly the origins of the posterior branches, determine how difficult the process will be.
Introduction
The nasoseptal flap (NSF) in its current form was first described by Hadad and Bassagestaguy 1 in 2006. There had been earlier reports of the use of narrow rectangular flaps of septal mucosa for the repair of cerebrospinal fluid (CSF) leaks 2, while others had described what were essentially rotational flaps with relatively random blood supplies. Hadad et al. designed a broad flap based on a known axial vessel that allowed more mucosa to be harvested with increased reliability; when combined with the relative ease of harvest this led to the wider adoption of the technique for skull base reconstruction with intraoperative CSF leak after transsphenoidal approaches to tumours 3 or for cerebrospinal fluid leak repair 4. In my hospital, the NSF is most often used for reconstruction of the previously irradiated nasopharynx after resection of recurrent nasopharyngeal carcinoma or after debridement of osteoradionecrosis 5. In a handful of cases, the flap pedicle is elongated by dissection to free the pedicle from the sphenopalatine foramen (SPF) for improved flap inset similar to the method described by Pinheiro-Neto 6. Shastri et al. 7 found that 5-6 cm of NSF elongation was possible in cadaveric dissections. This study used comprehensive anatomic dissections to determine how anatomical factors such as vessel coiling and branch configuration specifically influence elongation of the NSF pedicle.
Materials and methods
Dissections were conducted on tissue blocks containing a unilateral nasal cavity with adjoining maxillary sinus, pterygopalatine fossa (PPF) and the whole nasal septum taken from a total of 61 half-heads from embalmed cadavers that were being used for medical students teaching. Eight cadavers had been injected with a mixture of latex and Indian ink at the time of embalming. Materials were held under the Anatomy Act 1984 and procedures were approved by HM Inspector of Anatomy.
Microdissection technique
A set of 36 dissections were performed using a binocular Watson Barnet dissecting microscope (2.5X magnification) to completely delineate the pattern of arterial branching of the maxillary artery (MA) and the position of bony foramina in the PPF and around the SPF.
Endoscopic dissection technique
We examined the factors affecting mobility of the NSF pedicle through 25 dissections of the MA in the PPF using endoscopic instruments. First an NSF was harvested in the usual manner 3,4. A standard surgical marker was used to mark reference points after the SPF was exposed: marks were made along the anterior surface of the terminal MA at the SPF and at 12 and 6 o’clock positions of the SPF (as seen from the medial side). Factors limiting elongation and how they could be overcome in a step-wise fashion were elucidated. After each of the manoeuvres described below, we put the flap and artery on anterior stretch and measured the gain in pedicle length as the distance of the marking on the vessel from the markings on the SPF. During dissections all efforts were made to keep at least one of the SPF markings. Measurements were taken with a standard flexible surgical ruler throughout the dissections.
TETHER POINT 1: SPF AND POSTERIOR NASAL ARTERY (PNA)
The sphenopalatine artery (SPA) and its terminal branches are tightly adherent to the periosteum of the SPF that they exit through. The PNA then runs medially across the anterior sphenoid in between the sphenoid ostium and the superior rim of the posterior choana to reach the posterior part of the septum and is thus the pedicle of the NSF. The PNA travels downwards in the lateral nasal wall to enter bony canal(s) at the posterior end of the middle and inferior turbinates.
Release step 1A. Vessels are freed from the SPF: the mucosa anterior to the SPF is incised and elevated to reveal the foramen and the vessels that traverse it. The crista ethmoidalis is a useful landmark. The bone around the SPF can be removed by Kerrison Rongeurs to widen the orifice, and after the vessels have been dissected completely free from the SPF movement of the NSF pedicle will be possible with anterior traction.
Release step 1B. Transect the PNA at the SPF before it enters the turbinate canals. One study found that the palatal artery was the sole supply to the inferior turbinate in 1 of 40 specimens 8, potentially making this manoeuvre unnecessary although this variant was not encountered in this series.
TETHER POINT(S) 2: POSTERIOR BRANCHES
There are several branches – pharyngeal artery (PhA), artery to pterygoid canal (vidian artery), artery to the foramen rotundum – that enter the ostia in the posterior aspect of the PPF. While these bony foramina have fairly consistent locations in the superolateral corner of the posterior wall of the PPF, the vessels, and in particular the PhA, can arise from almost anywhere along the MA in the PPF. If the PhA arises ‘early’, that is it arises from the proximal part of the third part of the MA, then it will usually be long and generally not limit pedicle elongation before the palatine vessels do. On the other hand, if the PhA has an origin from the distal part of the MA (21%), where it often lies quite close to its bony foramen (palatovaginal canal) and thus also the SPF, then the short vessel will significantly hinder pedicle elongation. The vidian artery, and the artery to the foramen rotundum when it is present, also need to be divided for pedicle elongation, but generally shows much less variation in its origin compared to the PhA, i.e. ‘late’ vidian arteries are not found.
Release step 2. A ball probe is useful to dissect out the MA and its branches. Reflect the SPA anteriorly and downwards with some traction to reveal the posterior branches that are transected as they are encountered. Further bone removal above and below the SPF with Rongeurs or a drill may be needed; at least one of the reference points made by surgical marker is preserved (usually the 12 o’clock mark).
TETHER POINT 3: PALATINE BRANCHES
In the absence of tethering by posterior branches close to the SPF, the NSF pedicle can be advanced until limited by the entry of the palatine vessels into their bony canals.
Release step 3. Transect the palatine vessels before they enter their respective canals. This may be facilitated by expansion of the bony window inferiorly with Rongeurs which will improve exposure of the palatine vessels.
Tether points/release steps 2 and 3 are often closely intertwined, and sometimes it may be better to perform them in reverse order, depending on whether the posterior vessels are ‘early’ or ‘late’. ‘Complete dissection’ of the remainder of the MA in PPF did not significantly increase the pedicle length; transection of the infraorbital artery and posterior superior alveolar artery did not significantly influence mobilisation of the NSF pedicle.
Results
Pterygopalatine fossa coiling
The third part of the MA enters the PPF laterally at the pterygomaxillary fissure (PMF) and takes a convoluted path to exit at the SPF at the posterosuperior corner. There is more MA than ‘needed’ to cross from one side of the PPF to the other leading to arterial coiling; Shaheen 9 suggested that the degree of coiling was related to age. There is a variable amount of fat amongst the vessels; the nerve branches from the maxillary nerve form a layer posterior to the arteries whilst the relatively sparse veins tend to run anteriorly just under the periosteum.
In the terminal branch of the MA, the SPA divides into the posterior septal arteries (PSA) and PNA before the SPF in 89% of cases, as consistent with the literature 10. Thus, usually 2 or more arteries traverse the SPF and sometimes there are separate bony foramina for each branch 11.
Our analysis demonstrated that the vessel patterns could basically be grouped by the number of loops in the confines of the PPF; in simple terms, there were single-looped (SL) forms (19.2%) and double-looped (DL) forms (80.8%) (Fig. 1). The latter have been previously subdivided into M and E forms 10.
Gain in length of NSF pedicle
We looked at the gain in NSF pedicle length after the manoeuvres described above, and compared the results in both DL and SL forms. In DL forms, a total of 4.7 ± 0.5 cm of NSF pedicle elongation is possible after ‘full’ dissection/release of the MA in the PPF; elongation is limited to 2.7 ± 0.5 cm when only the artery at the SPF is dealt with, while 4.2 ± 0.6 cm is achieved after ligation of the greater palatine artery (GPA) and posterior branches. In SL forms, the corresponding figures are 2.6 ± 0.5 cm, 0.8 ± 0.3 cm and 2.3 ± 0.5 cm, respectively.
- The DL forms have a longer length of MA within the PPF, and in addition the distribution of the ‘laxity’ is slightly different between the different forms. The ‘extra’ (half) loop (over the SL form) usually lies between the GPA origin and the SPF and by virtue of this much more elongation is possible without GPA transection.
- Pedicle elongation is also affected by the location of the origin of the posterior branches, particularly the PhA and vidian artery. As discussed in ‘Tether point 2’, the PhA can branch from the proximal part of the third part of the MA in the PPF, and could be described as an ‘early’ PhA. On the other hand, if these arteries arise from the distal MA then they can be described as ‘late’ (Fig. 2). This latter variant, which occurs in 21% of cases, would branch off the MA close to the SPF and the palatovaginal/palatosphenoidal canal 12, and would be short and severely restrict pedicle movement until they are transected (Fig. 3). The ratio of ‘late’ versus ‘early’ PhA shows little difference amongst the different looped forms, although the limiting effect is likely to be much more pronounced in single-looped forms (18.2%). Interestingly, Pearson13 described the ‘early’ PhA as being aberrant found in 20% of cases in a study looking at transantral ligation; this was prior to the era of endoscopic nasal surgery.
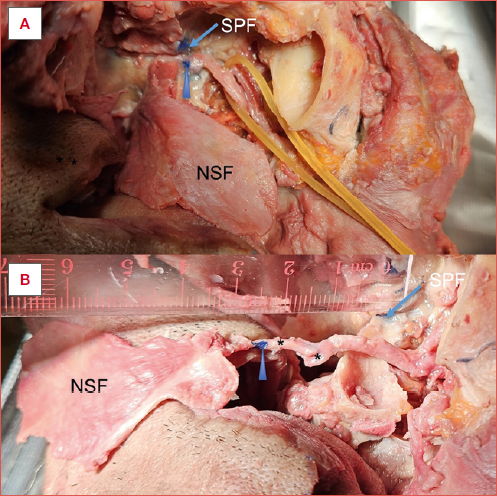
- In SL forms, most of the vessel length lies proximal to the GPA origin, and thus GPA transection is key to gaining significant pedicle length. However, the total length gain that can be achieved even with comprehensive dissection is very limited (2.6 ± 0.5 cm) in comparison to the DL form (Cover figure).
The results are summarised in Table I.
Discussion
The solid anatomical basis of the NSF 14,15, i.e. an axial pattern flap, makes it a reliable flap with a large area that can be moved about a wide angle of rotation based on its pedicle, and has become one of the most versatile intranasal flaps in terms of surface area and reach 16. Pinheiro-Neto et al. stated that the standard NSF can cover the anterior skull base 17 and reconstruct defects from expanded endoscopic approaches 18. However, under certain circumstances, coverage may still be insufficient 3,19 and in particular the anterior edge is most crucial 18,20. A cadaveric study 18 found that there was less than 5 mm overlap between the flap and anterior defect in 26.7% of cases, which would leave the integrity of the repair vulnerable following retraction/contraction of the flap. In clinical practice, they would add 6 mm when possible to account for this 17.
Some have pushed the limits of the design and strategies to both increase the flap surface area and the flap reach, flap extension 21,22 and flap (pedicle) elongation have been described. In an ‘extended NSF’, a flap area larger than the classic NSF is harvested to cover larger/wider defects; parts of the nasal floor and lateral nasal wall are incorporated, reliably nourished by the anastomoses between the septal arteries and the branches of the PNA that supply the lateral nasal wall. The ‘extended NSF’ is a straightforward modification, but by itself has little effect on the reach of the flap 21,22 and thus would not solve the problem with the anterior margin of the defect noted above.
In the elongated NSF, the flap pedicle is ‘lengthened’ or, more accurately, the coiled MA in the PPF is ‘straightened’, by freeing the vessel from the bony boundaries at the SPF, and dividing branches in the PPF, to allow the NSF to more reliably cover the anterior skull base including the posterior table of the frontal sinus as well as the oropharynx 23. Transoral robotic surgery defects are usually left to heal by secondary intention over 3-4 weeks, but it has become apparent that some oropharyngeal defects would benefit from reconstruction 24-26, e.g. to cover an exposed carotid artery, clips or bone, particularly if poor healing is anticipated (smokers, diabetics, or previous irradiation) or to reduce the time to adjuvant therapy. Turner et al. described the use of a transpalatal tunnel to bring the NSF to the oropharynx 23,27.
Prior studies into the MA pattern in the PPF 7,13,28,29 have mostly utilised dissections limited by a small surgical field that only partially reveals vessel patterns, making it difficult to appreciate all the factors and relationships influencing elongation. The comprehensive dissections used in this study, although somewhat ‘artificial’ in context of surgery, were vital in providing more complete information to properly guide the clinical application(s). This was followed by ‘surgical’ dissections with endoscopic instruments to demonstrate the anatomical factors limiting pedicle elongation. What this study clearly shows is that elongation is mostly influenced by 2 factors:
- MA coiling – the number of loops determines the actual vessel length in the PPF and thus how much the NSF pedicle can be elongated when straightened (Fig. 3). The exact classification or subtype of the arterial configuration described in previous publications is not as important as the simple recognition of the short SL variant.
- Branches such as the GPA, PhA and vidian artery can tether the main vessel at several key points. Surgical access to allow division of these branches requires significant dissection and bone removal. This is the main determinant of the ease of pedicle elongation. On the other hand, variable branch configurations, particularly ‘late’ vs ‘short’ PhA, will further influence the exact steps/sequence needed.
The results of the dissections found that up to 4.7 ± 0.5 cm additional pedicle length is available to the standard NSF, which can significantly widen its potential indications. This is less than but very similar to the findings of Shastri et al. 7.
We agree that the dissection can be technically challenging and there are concerns about an exposed pedicle. This study has expanded further on their findings in 2 important aspects. Firstly, the dissection required to elongate the pedicle by 2-3 cm is relatively straightforward being similar to SPA ligation for persistent epistaxis, while significantly more challenging dissection is needed to elongate the pedicle by an additional 2 cm requiring the identification and division of multiple branches in the confines of the PPF. Vessels and nerves would be at risk, and may increase the risk of flap necrosis or complications such as dry eye and infraorbital numbness. Secondly, and what has not been previously appreciated, is that this elongation may be severely limited (mean of 2.6 cm) in some variants (19.2%).
The standard NSF is very reliable 30, provided its limitations are respected. Partial flap necrosis may result from too much tension when used to cover the defects of borderline suitability. This would tend to cause venous insufficiency, which is more deleterious to flap survival than arterial insufficiency. It is important to maximise the venous return of the NSF by preserving a reasonable tissue bridge with the vessels, avoiding skeletonisation of the pedicle and too much tension on the pedicle. The most useful application of NSF pedicle elongation may be when it is used in an essentially ‘unplanned’ or ‘as needed’ manner, when a standard NSF has been harvested but the flap is just a bit too short to allow a satisfactory flap inset without tension. In such scenarios, it would seem preferable to avoid tension by performing an ad hoc limited pedicle dissection to lengthen the flap. Shastri regarded ‘full’ elongation as ‘excessive’ for anterior skull base defects 7. Vinceguerra et al. 31 described a series of 55 cases where elongated NSFs (described as ‘nasoseptal flaps with extended pedicle dissection’) were used for a variety of defects with 2 cases of partial/total necrosis.
The value of preoperative planning such as angiography is uncertain. Previous studies have not confirmed the utility of preoperative computed tomography angiography (CTA) in planning the standard NSF 32, but it could be of value when a fully-elongated NSF is planned for more distal defects 13 requiring more than 2-3 cm of elongation, or at the very least to exclude the existence of a short MA (i.e. SL form). However, CTA may not adequately visualise the smaller calibre vessels, particularly the posterior branches, and plain computed tomography may not adequately define the finer points of the bony architecture including foramina for more detailed planning. Moreover, CTA does not give information on veins.
Conclusions
This study has demonstrated that basically 2 factors underlie the anatomical basis of the elongated NSF – the looping of the IMA in the PPF determines the maximal length of the pedicle when straightened, while the branch configurations and the bony architecture including the foramina in the PPF determine how easy it is to utilise this length.
Potential pedicle elongation by up to 5 cm can significantly widen the utility of the NSF in regional reconstruction both anteriorly for the posterior table of the frontal sinus and inferiorly for oropharyngeal defects. However, only half of this elongation may be achievable in approximately 1 in 5 cases due to variations in anatomy (and requires more dissection within the PPF). It is suggested that limited ad hoc elongation (to gain 1-2 cm) to facilitate inset is preferable to overstretching/overextending the standard NSF. Further clinical experience and studies are needed to definitively demonstrate these (and other) points.
Acknowledgements
The author sincerely thanks those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude.
Conflict of interest statement
The author declares no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical consideration
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: May 31, 2022
Accepted: October 16, 2024
Figures and tables
Figure 1. Patterns of the third part of the maxillary artery (MA) obtained by complete dissection (vessels removed from PPF for clarity), showing DL forms – E form (A) and M form (B), and SL forms (C). The DL form has a greater length of artery allowing more potential elongation of the NSF pedicle. PSAA: posterior superior alveolar artery; PhA: pharyngeal artery; PSA: posterior septal artery; PNA: posterior nasal artery; IOA: infraorbital artery; DPA: descending palatine artery; SPA: sphenopalatine artery; VA: vidian artery.
Figure 2. Schematic to show the differences between double-looped (A) and (B) single-looped forms. Note that the segment of vessel in between the origin of the GPA and the SPF (grid pattern) is much longer in the DL form. When the distal MA/SPA has been freed from the SPF, then pedicle of the NSF can be pulled in an anterior direction. Much more elongation is possible in the DL form before being limited by the entry of the GPA into the palatine canal. GPA transection is much more important for pedicle elongation in the SL form.
Figure 3. Schematic to demonstrate how the origin of the pharyngeal artery (PhA, solid black) on the maxillary artery (MA) determines how long the vessel in the PPF will be, which in turn determines how much the distal part of the MA, i.e. the NSF pedicle, can be mobilised. A short ‘late’ PhA would tend to tether the pedicle close to the SPF hindering NSF pedicle mobilisation.
| Step 1 | Step 2 and 3 | Step 4 | |
|---|---|---|---|
| Open up SPF, transect PNA | GPA and posterior branch transection | Full dissection | |
| Single loop (n = 5) | 0.8 +/- 0.3 | 2.3 +/- 0.5 | 2.6 +/- 0.5 |
| Double loop (n = 21) | 2.7 +/- 0.5 | 4.2 +/- 0.6 | 4.7 +/- 0.5 |
| Gain in length of NSF pedicle (mean in cm +/- standard deviation SD) after stepwise manoeuvres. N = 26. GPA transection is proportionally more important in single looped form for pedicle elongation. Step 2 and 3 are grouped together as they may be performed in ‘reverse order’. | |||
References
- Hadad G, Bassagasteguy L, Carrau R. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882-1886. doi:https://doi.org/10.1097/01.mlg.0000234933.37779.e4
- Hirsch O. Successful closure of cerebrospinal fluid rhinorrhea by endonasal surgery. AMA Arch Otolaryngol. 1952;56:1-12. doi:https://doi.org/10.1001/archotol.1952.00710020018001
- Zanation A, Carrau R, Snyderman C. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy. 2009;23:518-521. doi:https://doi.org/10.2500/ajra.2009/23.3378
- Eloy J, Kalyoussef E, Choudhry O. Salvage endoscopic nasoseptal flap repair of persistent cerebrospinal leak after open skull base surgery. Am J Otolaryngol. 2012;33. doi:https://doi.org/10.1016/jamjoto.2012.07.005
- Lai C, Chow S, Tong M. Endonasal acoustic doppler sonography in predicting the survival of nasoseptal flap following previous irradiation. Laryngoscope. 2023;133:244-247. doi:https://doi.org/10.1002/lary.30095
- Pinheiro-Neto C, Paluzzi A, Fernandez-Miranda J. Extended dissection of the septal flap pedicle for ipsilateral endoscopic transpterygoid approaches. Laryngoscope. 2014;124:391-396. doi:https://doi.org/10.1002/lary.24256
- Shastri K, Leonel L, Patel V. Lengthening the nasoseptal flap pedicle with extended dissection into the pterygopalatine fossa. Laryngoscope. 2019;130:18-24. doi:https://doi.org/10.1002/lary.27984
- Al-Shouk A, Tatar I. The blood supply of the inferior nasal concha (turbinate): a cadaveric anatomical study. Anat Sci Int. 2021;96:13-19. doi:https://doi.org/10.1007/s12565-020-00552-0
- Shaheen O. Arterial epistaxis. J Laryngol Otol. 1975;89:17-34. doi:https://doi.org/10.1017/s002221510008004x
- Chiu T. A study of the maxillary and sphenopalatine arteries in the pterygopalatine fossa and at the sphenopalatine foramen. Rhinology. 2009;47:264-270. doi:https://doi.org/10.4193/Rhin08.153
- Wareing M, Padgham N. Osteologic classification of the sphenopalatine foramen. Laryngoscope. 1998;108:125-127. doi:https://doi.org/10.1097/00005537-199801000-00024
- Karligkiotis A, Volpi L, Abbate V. Palatovaginal (pharyngeal) artery: clinical implication and surgical experience. Eur Arch Otorhinolaryngol. 2014;271:2839-2843. doi:https://doi.org/10.1007/s00405-014-3111-6
- Pearson B, MacKenzie R, Goodman W. The anatomical basis of transantral ligation of the maxillary artery in severe epistaxis. Laryngoscope. 1969;79:969-984. doi:https://doi.org/10.1288/00005537-196905000-00014
- Chiu T, Shaw Dunn J. An anatomical study of the arteries of the anterior nasal septum. Otolaryngol Head Neck Surg. 2006;134:33-36. doi:https://doi.org/10.1016/j.otohns.2005.09.005
- Zhang X, Wang E, Wei H. Anatomy of the posterior septal artery with surgical implications on the vascularized pedicled nasoseptal flap. Head Neck. 2015;37:1470-1476. doi:https://doi.org/10.1002/hed.23775
- Kim S, Park H, Jeon S. Versatility of the pedicled nasoseptal flap in the complicated basal skull fractures. Auris Nasus Larynx. 2013;40:334-337. doi:https://doi.org/10.1016/j.anl.2012.07.013
- Pinheiro-Neto C, Prevedello D, Carrau R. Improving the design of the pedicled nasoseptal flap for skull base reconstruction: a radioanatomic study. Laryngoscope. 2007;117:1560-1569. doi:https://doi.org/10.1097/MLG.0b013e31806db514
- Pinheiro-Neto C, Ramos H, Peris-Celda M. Study of the nasoseptal flap for endoscopic anterior cranial base reconstruction. Laryngoscope. 2011;121:2514-2520. doi:https://doi.org/10.1002/lary.22353
- Virgin F, Baranano C, Riley K. Frontal sinus skull base defect repair using the pedicled nasoseptal flap. Otolaryngol Head Neck Surg. 2011;145:338-340. doi:https://doi.org/10.1177/0194599811404527
- Wardas P, Tymowski M, Piotrowska-Seweryn A. Hadad-Bassagasteguy flap in skull base reconstruction – current reconstructive techniques and evaluation of criteria used for qualification for harvesting the flap. Wideochir Inne Tech Maloinwazyjne. 2019;14:340-347. doi:https://doi.org/10.5114/wiitm.2018.79633
- Chen M, Wang S, Zhu Y. Use of a posterior pedicle nasal septum and floor mucoperiosteum flap to resurface the nasopharynx after endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2012;34:1383-1388. doi:https://doi.org/10.1002/hed.21928
- Peris-Celda M, Pinheiro-Neto C, Funaki T. The extended nasoseptal flap for skull base reconstruction of the clival region: an anatomical and radiological study. J Neurol Surg B Skull Base. 2013;74:369-385. doi:https://doi.org/10.1055/s-0033-1347368
- Turner M, Geltzeiler M, Ramadan J. The nasoseptal flap for reconstruction of lateral oropharyngectomy defects: a clinical series. Laryngoscope. 2022;132:53-60. doi:https://doi.org/10.1002/lary.29660
- de Almeida J, Park R, Genden E. Reconstruction of transoral robotic surgery defects: principles and techniques. J Reconstr Microsurg. 2012;28:465-472. doi:https://doi.org/10.1055/s-0032-1313762
- de Almeida J, Park R, Villanueva N. Reconstructive algorithm and classification system for transoral oropharyngeal defects. Head Neck. 2014;7:934-941. doi:https://doi.org/10.1002/hed.23353
- Genden E, Park R, Smith C. The role of reconstruction for transoral robotic pharyngectomy and concomitant neck dissection. Arch Otolarygnol Head Neck Surg. 2011;137:151-156. doi:https://doi.org/10.1001/archoto.2010.250
- Turner M, Geltzeiler M, Albergotti W. Reconstruction of TORS oropharyngectomy defects with the nasoseptal flap via transpalatal tunnel. J Robot Surg. 2020;14:311-316. doi:https://doi.org/10.1007/s11701-019-00984-5
- Morton A, Khan A. Internal maxillary artery variability in the pterygopalatine fossa. Otolaryngol Head Neck Surg. 1991;104:204-209. doi:https://doi.org/10.1177/019459989110400208
- Kwak H, Jo J, Hu K. Topography of the third part of the maxillary artery via the transantral approach in Asians. J Craniofac Surg. 2010;21:1284-1289. doi:https://doi.org/10.1097/SCS.0b013e3181e1b33c
- Soudry E, Psaltis A, Lee H. Complications associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015;125:80-85. doi:https://doi.org/10.1002/lary.24863
- Vinceguerra A, Turri-Zanoni M, Ferrari M. Int Forum Allergy Rhinol. 2024;14:1240-1244. doi:https://doi.org/10.1002/alr.23326
- Adappa N, Learned K, Palmer J. Radiographic enhancement of the nasoseptal flap does not predict postoperative cerebrospinal fluid leaks in endoscopic skull base reconstruction. Laryngoscope. 2012;122:1226-1234. doi:https://doi.org/10.1002/lary.23351
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1002 times
- PDF downloaded - 122 times



