Head and neck
Vol. 45: Issue 4 - August 2025
The Host-index in patients undergoing upfront surgery and free flap reconstruction for head and neck squamous cell carcinoma
Abstract
Objective. The Host-index (H-index) is a value obtained using blood laboratory parameters. Elevated H-index is a negative prognostic factor in patients with head and neck squamous cell carcinoma (HNSCC). The purpose of our study was to assess the prognostic impact of the H-Index in patients with locally advanced tumours who underwent reconstructive surgery with free flaps.
Methods. We performed a retrospective study on a cohort of patients referred to our center from January 2013 to October 2018. We assessed the prognostic role of H-index in terms of disease-free (DFS) and overall survival (OS).
Results. A total of 99 patients were studied, with a median age of 66 years. After a median follow-up of 46.5 months (range, 1.4-121.9), 5-year OS was 59.6% (CI 29.3-57.5) and 5-year DFS 47.5% (CI 16.7-49.2). The H-index showed a statistically significant correlation with a shorter DFS (HR 1.2, 95% CI 1.1-1.4, p = 0.006). No correlations were found between surgical complications and the H-index.
Conclusions. This study confirmed that the H-index is an independent prognostic factor for DFS in patients with HNSCC undergoing microvascular reconstructive surgery and should be used to better stratify the risk of mortality and recurrence, with the aim of improving patient management.
Introduction
The immune system and the inflammatory response have a crucial role in cancer promotion and progression. Indeed, avoiding immune destruction and tumour-promoting inflammation have been identified as cancer hallmarks 1. During carcinogenesis, several genetic alterations and the loss of normal cellular regulatory processes may lead to the formation of neoantigens. These can be recognised by the immune system, triggering its activation 2,3.
It is also known that tumour microenvironment (TME), made of both tumour cells and stromal cells, directly stimulates the activation of inflammation and the release of mediators (chemokines, cytokines, and prostaglandins) 4, which are also involved in migration, invasion, and metastasis 5. Understanding the role of the immune system during carcinogenesis and tumour progression paved the way for the introduction of immune checkpoint inhibitors which interfere with the immune escape mechanisms used by cancer cells, resulting in improved survival 6.
In recent years, there have been many attempts to correlate blood values and inflammatory markers with the prognosis of patients affected by head and neck squamous cell carcinoma (HNSCC) 7, through the development of prognostic models. The Host Index (H-index), described by Valero et al. in 2020 9,10, is obtained by using preoperative blood values on haemoglobin, neutrophils, lymphocytes, monocytes, and albumin; its correlation with prognosis of patients with HNSCC has already been demonstrated in several studies 10-12.
Specifically, high H-index values correlate with a reduction in disease-specific and overall survival (OS). In the literature, the H-index has been studied in HNSCC patients within specific primary sites (e.g., oral cavity). However, it has never been correlated with postoperative complications. Our study aimed to explore the prognostic impact of the H-index in HNSCC patients undergoing reconstructive surgery with free flaps.
Materials and methods
A retrospective observational study was conducted including a series of consecutive patients treated at the Unit of Head and Neck Surgery of the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano (Italy) between January 2013 and October 2018. All patients underwent upfront surgery for Stage II-IVa/b HNSCC, requiring free flap reconstruction. The following data were collected: age, gender, smoking, and alcohol consumption, disease site and subsite, histological grading, surgical margins, number of pathological lymph nodes, and lymph nodes with extranodal extension (ENE), lymphovascular invasion (LVI), and perineural invasion (PNI).
All patients were staged according to the 8th Edition of the AJCC (American Joint Committee on Cancer) classification 12.
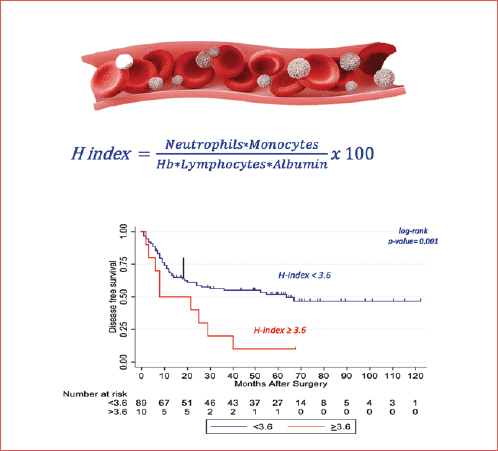
The following pretreatment blood test parameters were collected: haemoglobin, neutrophils, lymphocytes, monocytes, and albumin. The H-index was obtained using the following formula:
(Neutrophils×Monocytes)/(Haemoglobin×Lymphocytes×Albumin) ×100 12
Following international guidelines, patients underwent regular follow-up with endoscopic evaluation and radiological imaging every 1-3 months in the first and second year, and every 4-6 months for subsequent years for at least 5 years. A chest computed tomography (CT) scan was performed annually in previous and current smokers 13.
OS was defined as the time from surgery to death from any cause (event) or last follow-up (censored), disease-free survival (DFS) as the time from surgery to the first evidence of clinical recurrence (locoregional and/or distant) or death from any cause (event) or last follow-up (censored). Median follow-up was assessed with the reverse Kaplan-Meier method.
Univariate Cox regression models were performed including the following parameters: haemoglobin, lymphocytes, monocytes, neutrophils, neutrophil-to-lymphocyte ratio (NLR), pathological stage, surgical margins, ENE, LVI, PNI and H-Index. The results were reported in terms of hazard ratio (HR) and 95% confidence intervals (CI). All blood test parameters were considered as continuous variables. H-index was analysed as both a continuous variable and dichotomised as ≥ vs < 3.6, according to the study of Valero et al. 8.
We defined postoperative complications as adverse events requiring a surgical revision of the flap in the operating room. Mann-Whitney test was used to assess the difference in the H-index distribution between groups.
Variables found to be significantly associated with survival (i.e., OS and DFS) were included in a multivariable model according to the recommendations of Harrell et al. 14.
Survivals were estimated with the Kaplan-Meier method and compared using the log-rank test. Patients were stratified into three different risk categories: high risk (HR) patients with H-index ≥ 3.6 and with one or more negative prognostic factors (PNI, LVI, R1, ENE); intermediate risk (IR) patients with H-index ≥ 3.6 without negative prognostic factors or H-index < 3.6 and with one or more negative prognostic factors; low risk (LR) with H-index < 3.6 and no negative prognostic factors. Statistical analysis was performed using the Statistical Software for Data Science (STATA). A two-side p value ≤ 0.05 was considered statistically significant. The study was approved by the institutional ethical committee of the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano (local study identifier INT79/23). In accordance with national laws, no informed consent was required from the participants due to the retrospective nature of the study and the absence of identifying information.
Results
A total of 174 patients were identified. Detailed follow-up information were missing for 75 cases, and thus 99 patients were included. Median age was 66 years (range, 30-83 years). Most patients had loco-regionally advanced oral cavity SCC (87%), followed by oropharynx p16- (9%), hypopharynx (1%), and other sites (3%). Stage was II in 12%, III in 32% and IVa/b in 53%. Negative surgical margins were achieved in 70 patients (71%), and lymph node metastases with ENE were observed in 28 cases (28%). Adjuvant treatment (RT or CRT) was administered to 78 patients (79%).
The median follow-up was 46.5 months (range, 1.4-121.9). Five-year OS was 59.6% (CI 29.3-57.5) and 5-year DFS 47.5% (CI 16.7-49.2) (Figs. 1-2).
Forty-two patients died (42%), with the disease being the cause of death in 40 (95%). Twenty patients experienced local recurrence, 13 had local and regional recurrences, 6 patients had regional recurrences, and 9 had distant metastases.
Twenty-six patients (26%) experienced postoperative complications, specifically surgical revision of the flap in the operating room (Tab. I).
During follow-up, a second primary tumour was diagnosed in 5 patients. Median H-index was 1.6 (range, 0.4-9.2).
The H-index distribution did not differ between patients with or without surgical complications (p = 0.07). Higher H-index values were significantly associated with shorter DFS (HR = 1.2, 95% CI 1.1-1.4, p = 0.006), not with OS (HR = 1.2, 95% CI 0.9-1.3, p = 0.055).
H-index was ≥ 3.6 in 10 (10.1%) patients, and < 3.6 in the remaining 88 (88.9%) subjects. In the former group, 5-year DFS was 10% (95% CI 2-29) vs. 51.1% (95% CI 16-52) in the latter (p = 0.01). Five-year OS was 40% (95% CI 9.6-53.7) and 60% (95% CI 29.3-59) in subjects with H-index ≥ 3.6 and < 3.6, respectively (p = 0.5) (Fig. 3).
At univariate analysis, the only blood test parameter correlated with a significant increase of the HR for 5-year DFS was serum level of monocytes. None revealed a significant association with OS.
Disease parameters significantly associated with DFS at univariate analysis were stage, ENE, PNI, the presence of positive margins, and H-index. Factors associated with OS at univariate analysis were stage, ENE, and PNI (Tab. II).
Parameters included in the multivariable models were ENE, PNI, stage, and H-index.
At multivariable analysis for DFS, ENE, PNI and H-index were independently associated with DFS, while only PNI maintained a statistical significance with OS (Tab. III). Six HR patients (H-index ≥ 3.6 and +more than one negative prognostic factors) experienced disease recurrence. Among the 73 IR patients, 38 (38.4%) had recurrence, while only 3 of the 20 patients belonging to the LR group (14.2%) had disease relapse.
Discussion
Our results showed that the H-index is a prognostic factor for HNSCC patients treated with reconstructive surgery. A high H-index (≥ 3.6) was significantly associated with shorter DFS, but it did not predict OS.
At univariate analysis, pathological tumour stage, ENE, and PNI were significantly associated with worse OS and DFS, confirming their well-known negative prognostic value 15-17. Among the various blood parameters taken into account, only monocytes revealed a significant association with outcomes, but only at univariate analysis. This finding is in line with the role of monocyte derivatives (i.e., macrophages) in tumour progression, promoting the proliferation and survival of tumour cells, and lymphangiogenesis 18, consistent with what was highlighted in the study by Boscolo-Rizzo et al. 11.
H-index maintained an independent prognostic significance at multivariable analysis for DFS only, together with ENE and PNI. The only independent prognostic factor for OS that maintained significance at multivariable analysis was PNI.
In a study by Košec et al., several systemic inflammatory markers were identified as possible predictors of postoperative complications and poor survival in HNSCC patients undergoing reconstructive surgery with microvascular flaps 19. However, in our study, no association was found between the H-index and risk of postoperative complications.
We tried to stratify patients into different risk groups (HR, IR, LR) and observed that all patients with an H-index greater than 3.6 and with more than one negative prognostic factor experienced disease relapse. Though the results are not validated in terms of DFS, due to the small sample size, they are. The results are, however, very interesting and pave the way for new future studies.
Pre-treatment inflammatory and nutritional index values confirm their prognostic importance 20. Albumin is a surrogate marker of nutrition and an acute-phase protein. It has also been reported in the literature to be associated with increased postoperative morbidity and mortality, as well as more frequent surgical site infection or postoperative complications 21,22. For this reason, many studies recently underlined the importance of a proper preoperative boosting of the nutritional status of patients to be treated by major surgery, including those planned for microvascular reconstruction 23. Low levels of haemoglobin in peripheral blood in patients with HNSCC was also associated with tumour hypoxia, which induces radioresistance, compromises the effectiveness of treatments, and negatively influences prognosis with a substantial increase in the relative risk of mortality 24,25.
Drawbacks of this study include the limited sample size and the retrospective nature of analysis. Acknowledging these limitations, a high H-index (≥ 3.6) was associated with a higher risk of disease relapse, although further evaluation and confirmation on larger samples are advisable.
Conclusions
H-index is an easily retrievable tool composed of parameters routinely collected in the preoperative setting. Our study showed that H-index is a prognostic factor for DFS in HNSCC patients undergoing microvascular reconstructive surgery. Larger case series are needed to validate our findings.
Conflict of interest statement
All authors declare no conflicts of interest, except for Stefano Cavalieri. He declares occasional fees for participation as a speaker at conferences/congresses from AccMed; support for attending meetings and/or travel from AccMed, MultiMed Engineers srl, Care Insight sas.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
AD: conceptualization, supervision; AD, VC: methodology; VC, LG, SC, AD: writing – original draft preparation; AD, SC, LG, AA, FF, FI, MP: writing – review and editing; VC, LG: visualization. All authors have read and agreed to the published version of the manuscript.
Ethical consideration
This study was approved by the Institutional Ethics Committee (Fondazione IRCCS Istituto Nazionale dei Tumori of Milan) (approval number/protocol number INT79/23).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
In accordance with national laws, no informed consent was required from the participants due to the retrospective nature of the study and the absence of identifying information.
History
Received: August 8, 2024
Accepted: December 5, 2024
Figures and tables
Figure 1. Overall survival (5-year OS 59.6%).
Figure 2. Disease-free survival (5-year DFS 47.5%).
Figure 3. Overall survival stratified according to H-index cutoff of 3.6 (p = 0.5)
| Characteristic | N |
|---|---|
| Age median, range | 66 (30-83) |
| Gender | |
| Male | 69 (70%) |
| Female | 30 (30%) |
| Smoking (current/former) | 63 (63%) |
| Alcohol (current/former) | 14 (14%) |
| Pathologic grading | |
| G1 | 6 (6%) |
| G2 | 65 (65%) |
| G3 | 28 (28%) |
| Primary site | |
| Oral cavity | 86 (87%) |
| Oropharynx p16- | 9 (9%) |
| Hypopharynx | 1 (1%) |
| Other | 3 (3%) |
| Stage | |
| II | 12 (12%) |
| III | 32 (32%) |
| IVA | 38 (38%) |
| IVB | 17 (17%) |
| Extranodal extension (ENE) | 28 (28%) |
| Surgical margins (R1/R2) | 29 (29%) |
| Perineural invasion (PNI) | 49 (49%) |
| Lymphovascular invasion (LVI) | 31 (31%) |
| Surgical complications | 26 (26%) |
| Adjuvant treatment | |
| Radiotherapy (RT) | 40 (40%) |
| Chemoradiotherapy (CRT) | 38 (38%) |
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Stage (IV vs II-III) | 5.11 (1.22-21.50) | 0.026 | 4.35 (1.34-14.19) | 0.015 |
| ENE (present vs absent) | 2.11 (1.12-3.96) | 0.02 | 2.15 (1.22-3.76) | 0.007 |
| Margins (R1/R2 vs R0) | 1.24 (0.64-2.40) | 0.518 | 1.71 (0.97-3.02) | 0.062 |
| PNI (present vs absent) | 1.92 (1.02-3.60) | 0.042 | 2.02 (1.15-3.53) | 0.014 |
| Monocytes 103μL (continuous variable) | 4.18 (0.84-20.83) | 0.081 | 4.65 (1.01-21.49) | 0.049 |
| H-index (continuous variable) | 1.15 (0.99-1.34) | 0.055 | 1.22 (1.06-1.41) | 0.006 |
| LVI (present vs absent)) | 1.31 (0.69-2.48) | 0.402 | 1.46 (0.84-2.55) | 0.174 |
| Neutrophils 103μL (continuous variable) | 1.06 (0.91-1.24) | 0.458 | 1.14 (0.97-1.34) | 0.105 |
| Lymphocytes 103μL (continuous variable) | 0.82 (0.49-1.37) | 0.453 | 0.90 (0.60-1.37) | 0.645 |
| Albumin (continuous variable) | 0.54 (0.23-1.24) | 0.148 | 0.55 (0.25-1.19) | 0.132 |
| Haemoglobin (continuous variable) | 0.91 (0.72-1.13) | 0.414 | 1.01 (0.82-1.25) | 0.885 |
| Overall survival | Disease-free survival | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| ENE (present vs absent) | 1.97 (0.97-4) | 0.06 | 2.15 (1.15-4.01) | 0.016 |
| PNI (present vs absent) | 2.53 (1.26-5.09) | 0.009 | 2.60 (1.42-4.74) | 0.002 |
| Stage (IV vs II-III) | 2.87 (0.65-12.79) | 0.165 | 2.51 (0.74-8.54) | 0.142 |
| H-index (continuous variable) | 1.15 (0.99-1.34) | 0.068 | 1.26 (1.08-1.47) | 0.003 |
| H-index considered as continuous variable. | ||||
References
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31-46. doi:https://doi.org/10.1158/2159-8290.CD-21-1059
- Xavier F, Silva J, Rodini C. Mechanisms of immune evasion by head and neck cancer stem cells. Front Oral Health. 2022;3. doi:https://doi.org/10.3389/FROH.2022.957310
- Mantovani A, Allavena P, Sica A. Cancer-related inflammation. Nature. 2008;454:436-444. doi:https://doi.org/10.1038/nature07205
- Wang H-C, Chan L-P, Cho S-F. Targeting the immune microenvironment in the treatment of head and neck squamous cell carcinoma. Front Oncol. 2019;9. doi:https://doi.org/10.3389/fonc.2019.01084
- Mukaida N, Sasaki S, Baba T. Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. Mediators Inflamm. Published online 2014. doi:https://doi.org/10.1155/2014/170381
- Trivedi S, Sun L, Aggarwal C. Immunotherapy for head and neck cancer. Hematol Oncol Clin North Am. 2021;35:1021-1037. doi:https://doi.org/10.1016/j.hoc.2021.05.010
- Li K, Zeng X, Liu P. The role of inflammation-associated factors in head and neck squamous cell carcinoma. J Inflamm Res. 2023;16:4301-4315. doi:https://doi.org/10.2147/JIR.S428358
- Valero C, Zanoni D, Pillai A. Host factors independently associated with prognosis in patients with oral cavity cancer. JAMA Otolaryngol Head Neck Surg. 2020;146. doi:https://doi.org/10.1001/jamaoto.2020.1019
- Sansa A, Valero C, Holgado A. External validation of the H-index (host index) in patients with head and neck squamous cell carcinomas. Head Neck. 2023;45:178-186. doi:https://doi.org/10.1002/HED.27224
- Tomasoni M, Piazza C, Deganello A. The prognostic-nutritional index in HPV-negative head and neck squamous cell carcinoma treated with upfront surgery: a multi-institutional series. Acta Otorhinolaryngol Ital. 2023;43:170-182. doi:https://doi.org/10.14639/0392-100X-N2358
- Boscolo-Rizzo P, Zanelli E, Giudici F. Prognostic value of H-index in patients surgically treated for squamous cell carcinoma of the larynx. Laryngoscope Investig Otolaryngol. 2021;6:729-737. doi:https://doi.org/10.1002/lio2.603
- Amin M. AJCC Cancer Staging Manual. Springer International Publishing; 2017.
- Caudell J, Gillison M, Maghami E. NCCN guidelines insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20:224-234. doi:https://doi.org/10.6004/jnccn.2022.0016
- Harrell F, Lee K, Mark D. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. doi:https://doi.org/10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
- Quintana D, Dedivitis R, Kowalski L. Prognostic impact of perineural invasion in oral cancer: a systematic review. Acta Otorhinolaryngol Ital. 2022;42:17-25. doi:https://doi.org/10.14639/0392-100X-N1653
- Burusapat C, Jarungroongruangchai W, Charoenpitakchai M. Prognostic factors of cervical node status in head and neck squamous cell carcinoma. World J Surg Oncol. 2015;13. doi:https://doi.org/10.1186/S12957-015-0460-6
- Bradley P, MacLennan K, Brakenhoff R. Status of primary tumour surgical margins in squamous head and neck cancer: prognostic implications. Curr Opin Otolaryngol Head Neck Surg. 2007;15:74-81. doi:https://doi.org/10.1097/MOO.0b013e328058670f
- Jahandideh A, Yarizadeh M, Noei-Khesht Masjedi M. Macrophage’s role in solid tumors: two edges of a sword. Cancer Cell International. 2023;23:1-25. doi:https://doi.org/10.1186/S12935-023-02999-3
- Košec A, Solter D, Ribić A. Systemic inflammatory markers as predictors of postoperative complications and survival in patients with advanced head and neck squamous cell carcinoma undergoing free-flap reconstruction. J Oral Maxillofac Surg. 2022;80:744-755. doi:https://doi.org/10.1016/j.joms.2021.12.011
- Boscolo-Rizzo P, D’Alessandro A, Polesel J. Different inflammatory blood markers correlate with specific outcomes in incident HPV-negative head and neck squamous cell carcinoma: a retrospective cohort study. BMC Cancer. 2022;22. doi:https://doi.org/10.1186/s12885-022-09327-4
- Lim W, Roh J, Kim S. Pretreatment albumin level predicts survival in head and neck squamous cell carcinoma. Laryngoscope. 2017;12. doi:https://doi.org/10.1002/lary.26691
- Xu H, Han Z, Ma W. Perioperative albumin supplementation is associated with decreased risk of complications following microvascular head and neck reconstruction. J Oral Maxillofac Surg. 2021;79:2155-2161. doi:https://doi.org/10.1016/j.joms.2021.04.030
- Bertazzoni G, Testa G, Tomasoni M. The Enhanced Recovery After Surgery (ERAS) protocol in head and neck cancer: a matched-pair analysis. Acta Otorhinolaryngol Ital. 2022;42:325-333. doi:https://doi.org/10.14639/0392-100X-N2072
- Lazzari G, Silvano G. From anemia to erythropoietin resistance in head and neck squamous cell carcinoma treatment: a carousel driven by hypoxia. Onco Targets Ther. 2020;13:841-851. doi:https://doi.org/10.2147/OTT.S242263
- Birgegård G, Aapro M, Bokemeyer C. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68:3-11. doi:https://doi.org/10.1159/000083128
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 928 times
- PDF downloaded - 265 times



