Rhinology
Vol. 45: Issue 4 - August 2025
Ecchordosis physaliphora of the clivus: a case series and narrative review of the literature
Abstract
Background. Ecchordosis physaliphora (EP) is a benign hamartomatous lesion, most commonly found in a retroclival position, as an incidental radiological finding. EP may become symptomatic, by creating a clival defect, leading to CSF leak and meningitis, thus requiring surgical treatment.
Methods. Retrospective case series.
Results. We present 5 cases of retroclival EP (4 males, 1 female; age range, 34-81 years) presenting in our department over the last 6 years. Four patients presented with CSF leak; 3 also had a history of bacterial meningitis, while one was diagnosed with meningitis on presentation. One patient was and remains asymptomatic 28 months later with the lesion being an incidental finding.
The EP was treated in all 4 symptomatic patients with removal of the lesion and reconstruction of the defect with an endoscopic endonasal transclival approach (EETTA). All 4 patients remain free of symptoms while the lesion has not recurred for 81, 72, 52, and 22 months, respectively.
Conclusions. A review of the literature depicts that there is a shift from transcranial approaches to the EETTA for treating retroclival EP. The present case series highlights that EETTA facilitates the complete excision of EP lesions, as well the reconstruction of the resulting defects, with minimal morbidity and hospitalisation.
Introduction
Ecchordosis physaliphora (EP) is a benign lesion arising from remnant cell pools of the embryonic notochord 1. It can be located anywhere along the craniospinal axis, but most commonly is found either in the sacrococcygeal or in the retroclival area 2. During the third week of gestation the foetal notochord develops as the foetal axial skeleton in vertebrates. It starts to regress around the sixth week and by the first year of life it has completely disappeared. Some remnants can still be found in adult intervertebral discs, contributing to the development of the nucleus pulposus3. However, the notochord may assume variable extensions toward its caudal and cephalic ends, resulting in heterotopic notochordal tissue remnants, usually in the sacrococcygeal and upper clivus region, explaining the predisposition of notochord deriving lesions for these areas in adult life4,20.
In most cases EP is presented as a retroclival extraosseous intradural gelatinous lesion, inside the prepontine cistern, near the Dorello’s canal 1-4,17. It may also be presented with a filiform, osseous/cartilaginous attachment, its famous hallmark stalk or pedicle, to the posterior clival wall 1-4,16. This presentation of the EP results from the migration pathway of physaliphorous cells, which originate intraclivaly, penetrate the dorsal clival wall, then the dura mater and even enter the subarachnoid space 1-4. This is the reason why physaliphorous cells can be found in all of these spaces and in some cases EP can manifest as solely intradural, extradural retroclival or clival lesions and even penetrate the sphenoid sinus1-4 .
Commonly EP is asymptomatic, and it was discovered incidentally in about 0.5-2% of autopsies 1 and in up to 8% of imaging studies as an incidental finding 18,19. Usually, its size ranges from a few mm to 0.5 cm diameter, but cases of gigantic EP of up to 2-3 cm have been reported 5-7. In most cases it is an asymptomatic lesion, rendering diagnosis very difficult.
In rare occasions, it can induce symptoms, by means of pressure to the pons or the abducens nerve, especially in its intradural prepontine localisation 1. The most common symptoms include headache, pneumocephalus, spontaneous cerebrospinal fluid (CSF) rhinorrhoea with meningitis, and abducens nerve palsy 1,8-15. When it presents as a predominantly clival lesion, it can lead to a defect of the posterior wall of the sphenoid sinus and the dura mater, CSF leak and even bacterial meningitis.
The present case series reports on 5 cases of retroclival EP, 4 of them presenting with CSF leak and with acute bacterial meningitis. Symptomatic patients were all managed with an endoscopic endonasal transphenoidal transclival approach for lesion excision and contextual multilayer skull base reconstruction. Imaging, perioperative and histological details of the cases leading to the diagnosis of EP are highlighted and a literature review of the reported cases of EP is included. The challenging differential diagnosis of EP from chordoma is also thoroughly discussed.
Materials and methods
Retrospective study of case files and data of 4 patients with symptomatic EP, who were treated surgically with an endoscopic endonasal transsphenoidal / transclival approach, with subsequent multilayer contextual reconstruction of the resulting skull base defect, and one asymptomatic patient, whose EP was an incidental finding.
Also, a narrative review of the literature was carried out using the PubMed/MEDLINE database, for the term “ecchordosis physaliphora”, with focus on case studies and series reporting on patients with surgically treated EP.
Results
Case reports
PATIENT 1
An 81-year-old male patient with no previous medical history presented with headache, nuchal rigidity and fever. Lumbar puncture confirmed bacterial meningitis, and he was treated with intravenous antibiotics. He improved and one week later, asymptomatic, he was discharged with per os antibiotics. However, he was readmitted 5 days later with the same symptoms of recurrent bacterial meningitis. During the second admission he also presented with CSF leak from his left nostril. High resolution CT depicted opacification of the sphenoid sinus and a well circumscribed defect of the posterior wall of the sphenoid at the midline clivus. The patient was referred to our department for ENT evaluation. Evaluation of MRI scans that the patient had from the first admission revealed a homogeneous T2W hyperintense cystic lesion of the posterior wall of the sphenoid arising from the prepontine cistern. That lesion, presenting with common symptoms of EP and typical findings on MRI, was missed during the first admission of the patient (Fig. 1).
PATIENT 2
A 60-year-old male patient was admitted to another hospital with acute bacterial meningitis that was treated with intravenous antibiotics. He also had a history of rhinorrhoea lasting 15 days that was diagnosed and treated as a common cold. On CT scan an opacification of the sphenoid sinus was noticed as well as a bony defect of the posterior wall of the sphenoid. The patient was referred to our department for evaluation. CT and MRI scans demonstrated a well-defined midline cystic mass, 1 cm in maximal diameter, originating in the prepontine cistern, eroding the middle third clivus and extending into the posterior sphenoid sinus. It also presented with a stalk-like connection to the clivus. The lesion was hypointense in T1W and hyperintense in T2W images, there was no enhancement after gadolinium contrast administration, and it was in contact to the basilar artery. The radiologic findings were suggestive of EP-related CSF leak complicated with bacterial meningitis (Fig. 2).
PATIENT 3
A 64-year-old female with a history of intermittent watery rhinorrhoea since 2013, for which she was treated with topical corticosteroids as allergic rhinitis, was referred to our department for evaluation after a single episode of meningitis. Nasal fluid sample was positive beta-2 transferrin confirming the presence of CSF. Brain CT and MRI showed a lesion occupying the sphenoid sinus causing bony erosion of the posterior wall of the sphenoid and the clivus. The lesion was hypointense in T1W and hyperintense in T2W images and did not enhance with contrast administration. Radiological findings were suggestive of EP complicated with CSF leak and bacterial meningitis (Figs. 3 and 4).
PATIENT 4
A 53-year-old patient with a 7 year history of left sided watery rhinorrhoea was referred to our department for evaluation after an episode of bacterial meningitis. Rhinorrhoea was intermittent, stopping for weeks and then recurring, exacerbated by leaning forward and leaving typical watery spots on the pillow overnight. Nasal fluid sample was positive for beta-2 transferrin. MRI of the brain with contrast revealed a prepontine non-enhancing lesion, along the dorsal aspect of the clivus that was T2 hyperintense and T1 isointense with intermediate signal on diffusion sequences. Resection via an endoscopic endonasal transphenoidal transclival approach and appropriate reconstruction of the clival defect revealed a benign lesion. This lesion was an EP (Fig. 5).
PATIENT 5
A 34-year-old patient with chronic rhinosinusitis with nasal polyps and a history of Crohn’s disease, Cogan’s syndrome and ankylosing spondylitis underwent MRI and CT as a follow-up for chronic rhinosinusitis. A lesion hypointense in T1, hyperintense in T2 with no contrast enhancement, located in the retroclival prepontine region, an incidental imaging finding in an asymptomatic patient was discovered. This lesion had all the typical features of EP. CT performed with contrast administration revealed a bony clival defect, a well demarcated smoothly corticated region, with no aggressive characteristics. The patient is still in follow-up as the lesion has not resulted in clival defect and CSF leak and does not cause compression of crucial structures, like the brainstem or cranial nerves due to its small size (maximum lesion diameter, 8 mm).
The management, surgical treatment and intraoperative findings of patients 1 through 4 were very similar. An endoscopic endonasal transphenoidal/transclival approach was applied with subsequent multilayer contextual reconstruction of the resulting skull base defect. First, a nasoseptal (Hadad-Bassagaisteguy) flap was harvested and stored in the nasopharynx. The anterior wall, intersphenoid septum, bony septa and rostrum of the sphenoid were dissected and completely drilled out, as well as the posterior third of the nasal septum. These steps are necessary to achieve good visualisation and accessibility of the posterior sphenoid wall and clivus. A greyish/whitish cystic pulsating lesion was identified that protruded into the sphenoid sinus through the upper clivus. The mass was well circumscribed, and it was easily dissected from the surrounding bone. No adhesions or infiltration of the mass to the bone were identified after sending part of the mass for histology, the remaining was cauterised with bipolar diathermy and then excised, resulting in round smooth defect in the clivus and the dura mater was visualised, through which CSF leaked. Right posterior to the defect, the basilar artery was clearly identified. The surrounding bone of the sphenoid, including the sphenoid septa, was drilled and the surrounding mucosa of the posterior sphenoid sinus was removed in order to place the nasoseptal flap. For the reconstruction of the defect a 3-layer technique was used. First, fat harvested from the patient’s thigh was used to fill the bony defect of the clivus. Then, iliotibial tract fascia was used as an overlay graft, between the dura mater and the bone. Last, the nasoseptal flap, that was initially stored in the nasopharynx, was rotated and put in the posterior wall of the sphenoid, covering the whole defect. The whole reconstruction was sealed with tissue glue (DurasealTM) and the sphenoid sinus packed with dissolvable material (GelfoamTM and SurgicelTM).
Histologic examination of the lesions demonstrated the characteristic physaliphorous cell with the large intracytoplasmic vacuoles, gathering in clusters, in a hypocellular myxomatous matrix of extracellular mucin (Fig. 6). There was absence of mitosis or atypia and sparse pleomorphism. There was no evident bone infiltration or necrosis in histology. All these findings are consistent of a benign tumour of notochordal origin, most probably EP. Moreover, the various infiltrations of histiocytes, plasmatocytes and lymphocytes, as well as haemorrhagic findings could be related to local infections of the lesion and possibly even suggest a mechanism of inflammatory dehiscence of the ecchordosis along its migratory pathway through the clivus and dura mater, leading to the communication defect.
Immunohistochemistry demonstrated heterogeneous epithelial membrane antigen (EMA) and S-100 positivity and local cytokeratin AE1/AE3 and cytokeratin-8 positivity, all consistent with notochordal deriving lesions.
The postoperative course of the patients was uneventful, with no postoperative complications. Lumbar drainage was not used for any of the patients postoperatively. Hospitalisation time ranged from 2 to 4 days (mean, 3 days).
Following the surgery all patients have remained asymptomatic for 81, 72, 52, and 22 months, respectively. Patient 5, who did not meet criteria for surgery, remains in follow-up, and is still asymptomatic 28 months after diagnosis.
Discussion
A total of 45 cases of symptomatic, surgically treated EP have been reported in the literature to date 1-7,9-15,18,20-38,40. The symptoms, from a pathophysiologic standpoint, are associated with compression of the pons and cranial nerves, obstruction of ventricular canals, infection, haemorrhage within the lesion and defects in the dura mater and clival bone. EP related symptoms in the literature include headache, dizziness, diplopia due to abducens nerve palsy, facial pain and dysaesthesia due to trigeminal nerve pressure, gate instability and hemiparesis, related either to compression of the pons or to not communicating hydrocephalus. Cases of fatal subarachnoid haemorrhage, due to bleeding EP, have also been reported, as well as cases of tension pneumocephalus 14,21,22. However scant the literature results on EP are, despite its slow growing and indolent nature, this lesion can lead to serious debilitating or even life-threatening complications.
Interestingly, increasingly more cases of EP related to CSF rhinorrhoea and meningitis are being reported in the last 10 years 1,2,9,10,12,23,24,26. Actually, CSF rhinorrhoea with or without meningitis is the most common reported EP related complication in the recent literature 1. The reason seems to be the modern imaging machines and advanced modalities of MRI that help discover the lesions and better differentiate EP from other skull base masses, such as chordoma and benign notochordal cell tumours (BNCT). Moreover, the endoscopic endonasal transphenoidal transclival approach (EETTA) has become prevalent for the management of midline skull base tumours, such as meningiomas, chordomas, craniopharyngiomas and epidermoid cysts and, as reported in the literature, it is the surgical approach of choice for the management of CSF leaks and EP management as well 1-3,9,10,12,14,23-26. EETTA allows to directly visualise and access the clival and retroclival region, allowing for safer and total excision of the tumour, as well as reconstruction of the resulting defects with minimal morbidity for the patient. Therefore, biopsy and definitive diagnosis of these masses have become more feasible thanks to EETTA1,9,23,24,26. In the past such cases would have been treated conservatively, or even if operated a biopsy of the mass may have not been made possible. Several different surgical approaches have been used in the past, such as suboccipital, retrolabyrinthine presigmoid, frontotemporal, endoscopic trans-third ventricle, transoral, transpalatal and transmaxillary approaches. However, EETTA seems to be safer, with reduced morbidity and shorter hospitalisation 1,9,23,24,26.
Chordoma and BNCT share a common embryological origin with EP and thus many similarities in histological patterns, immunohistochemical staining, location and sometimes imaging findings in radiology. Thus, differentiating among these entities can be challenging, although necessary, since they differ in prognosis, malignant and metastatic potential and treatment strategies 18.
As delineated in the literature, EP most commonly presents on MRI as a cystic, smooth, prepontine lesion, hypointense to brain parenchyma in T1W images and homogeneously hyperintense in T2W images, with no contrast enhancement on contrast administration and no to minimal diffusion restriction in DWI 16,23,24. CT is generally not as sensitive for such lesions, due to the posterior fossa artifacts and the near CSF density of the EP 18. A smooth well corticated clival defect in the dorsal aspect of the clivus may be identified, as well as the hallmark osseous stalk of the EP that connects the intradural to the extradural clival part of the mass 18,24. These imaging characteristics stem from the high water, low lipid, low protein component of EP and the lack of aggressive infiltrative growth pattern.
On the other hand, chordomas appear hyperintense in both T1W and T2W images with an inhomogeneous contrast enhancement 16,23,24. They are predominantly presented as extradural intraosseous clival tumours, with an infiltrative, lytic destructive pattern of the clival bone and intratumoural calcifications, as shown on CT 23. These differences set for a clear differential diagnosis between EP and chordoma on imaging findings alone 1,23. However, in the rare instances of intradural chordoma the imaging findings are less distinct from those of an EP or a BNCT, which all may appear similar on MRI and CT 17,18. Therefore, an intradural, prepontine large mass, that appears hypo- to isointense on T1W and iso- to hyperintense in T2W images, with an inhomogeneous intensity and low inhomogeneous contrast enhancement could represent either an intradural chordoma, a BNCT, or a giant EP. Also, intradural chordoma, in contrast to the classic extradural intraosseous counterpart, shows a more favourable prognosis, slower growth pattern, less invasive/malignant nature and no bone involvement 3. From a clinical standpoint, these masses should be managed surgically once they become symptomatic or suspicious on imaging and a long-term postoperative follow-up with MRI is necessary to ensure no recurrence of the lesion 16.
The histological and immunohistochemical examination of EP and chordoma share many similarities and in certain cases fail to differentiate between the two 1,2,14. The physaliphorous cells are the hallmark histological finding in all notochord derived lesions and they are empty looking round or polygonal cells, with large mucinous intracytoplasmic vacuoles, eosinophilic cytoplasm and small round central or eccentric nuclei organised in lobules, chords or sheets inside a myxoid amphiphilic matrix 1,14. On immunohistochemical examination all notochord derived lesions are positive for vimentin, low molecular weight cytokeratin, EMA, and S-100 protein 13,18. In EP, the physaliphorous cells are organised in aggregates and there is absence of hypercellularity, mitosis, atypia and pleomporhism 1,4. Also, there is no tissue infiltration on the specimen’s borders and the MIB-1, Ki-67 index is < 1% 9. On the contrary, in chordoma, physaliphorous cell aggregates form cords and lobules, as they are separated by fibrous septa and there is vast extracellular myxoid tissue 1. Typical finding of malignancy, such as atypia, mitosis, hyperchromatism, hypercellularity, pleomorphism and necrosis are also present in chordoma 1. In chordoma immunostaining is positive for S-100, vimentin, CK 8/18, CK19, AE1-AE3, CK903, EMA, glycogen, neural-type cadherin, 5 nucleotidase, CEA and lysozyme, while it is negative for chromogranin, CK7, CK20 14. Moreover, in chordoma MIB-1, Ki-67 index is > 2, thus representing the most pathognomonic tool in differentiating chordoma from EP 13. In addition, the histological and immunohistochemical patterns described above are not always typically present in the biopsy specimens, especially when the mass is not excised as a whole, but rather partially excised.
It is evident that the diagnosis of EP against chordoma cannot always be established on pathology alone, but clinical, intraoperative and imaging findings must all be evaluated 1,18. In the end, it is the long-term follow-up with MRI that will prove the benign nature of lesion, in terms of no recurrence. In a systematic review published in 2023 by Stuebe et al. 39, it was found that the average reported follow-up period after surgery for EP was 19.5 ± 17.2 months, while the mean time to clival chordoma recurrence was 53.9 ± 26.8 months 39. This highlights the need to follow-up all lesions, even if suspected to be benign, for as long as possible, to rule out chordoma. Ideally, the follow-up period of EP, should be longer than the mean time of chordoma recurrence, which is reported to be 4.5 years (54 months) in the literature 39. In the present case series all 4 patients that were operated on remain asymptomatic with no recurrence of tumour for 81, 72, 52, and 22 months, respectively. Patient 5, who did not meet criteria for surgery, remains in follow-up, and is still asymptomatic 28 months later. The follow-up periods reported herein, especially regarding patients 1-3, are among the longest reported in the literature 39, a fact that supports the diagnosis of EP over BNCT or low malignance chordoma.
Conclusions
EP represents a rare congenital benign ectopic notochordal remnant, usually located in the retroclival prepontine region. Most commonly, it is clinically an inconspicuous lesion that appears in 0.5-2% of autopsies. However, EP may infrequently turn into a symptomatic lesion and be associated with mass effect due to its extension to surrounding crucial anatomical structures − brainstem or cranial nerves − or with CSF leak with recurrent meningitis due to clival defect. In these cases, surgical treatment is imperative.
This paper highlights the management of 5 patients affected by EP. The aim of this study is to underline the importance of timely diagnosis of this rare entity as it can easily be missed due to its small size and rarity.
An endoscopic endonasal transphenoidal/transclival approach facilitates the complete excision of these lesions, as well as the 3-layer reconstruction of the skull base defects, with minimal morbidity.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
CG: conceptualization, supervision; PP, GEP, AO, VC, AL, IG, CG: patient data collection and preparation and literature review; PP: writing.
All authors have read and agreed to the final version of the manuscript.
Ethical consideration
As this study was a retrospective study of patients’ records, the approval of the Ethical committee of the Scientific Council of Hygeia Hospital was requested and was granted (20/3/2024). No patient is identified or identifiable from the data collected or submitted. Written informed consent was obtained from each patient for data publication. The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
History
Received: April 10, 2024
Accepted: January 5, 2025
Figures and tables
Figure 1. Axial plane CT of patient 1 showing opacification of the sphenoid sinus and a well circumscribed defect of the posterior wall of the sphenoid at the midline clivus (white arrow).
Figure 2. Axial plane CT (left) and MRI T2W (right) of patient 2 showing a well-defined midline cystic mass, 1 cm in maximal diameter, originating in the prepontine cistern (black arrow), eroding the middle third of the clivus (white arrow) and extending into the posterior sphenoid sinus.
Figure 3. Sagittal (left) and axial (right) planes of MRI in patient 3, showing a T2W hyperintense lesion causing erosion of the posterior sphenoid sinus wall and clivus, leading to CSF leak.
Figure 4. Perioperative identification of the defect in the posterior sphenoid sinus wall in patient 3 using navigation system (upper left coronal, upper right sagittal, lower left axial views, lower right endoscopic view of the lesion in the posterior sphenoid sinus wall).
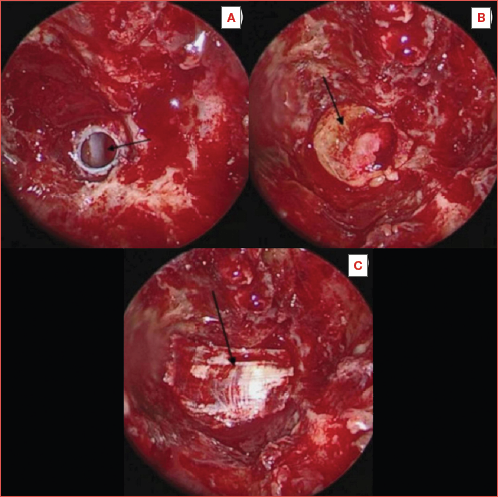
Figure 5. Perioperative identification of the lesion and defect in the posterior wall of the right sphenoid sinus in patient 4.
Figure 6. Histopathology stains: tumour cells with vacuolated cytoplasm (physaliphorous cells) disposed randomly showing epithelial membrane antigen and S100 protein positivity (black arrows) on a myxoid matrix.
References
- Veiceschi P, Arosio A, Agosti E. Symptomatic ecchordosis physaliphora of the upper clivus: an exceedingly rare entity. Acta Neurochir (Wien). 2021;163:2475-2486. doi:https://doi.org/10.1007/s00701-021-04857-5
- Dias L, Nakanishi M, Mangussi-Gomes J. Successful endoscopic endonasal management of a transclival cerebrospinal fluid fistula secondary to ecchordosis physaliphora—an ectopic remnant of primitive notochord tissue in the clivus. Clin Neurol Neurosurg. 2014;117:116-119. doi:https://doi.org/10.1016/j.clineuro.2013.11.026
- Choudhri O, Feroze A, Hwang P. Endoscopic resection of a giant intradural retroclival ecchordosis physaliphora: surgical technique and literature review. World Neurosurg. 2014;82:912.e21-912.e9.12E26. doi:https://doi.org/10.1016/j.wneu.2014.06.019
- Alli A, Clark M, Mansell N. Cerebrospinal fluid rhinorrhea secondary to ecchordosis physaliphora. Skull Base. 2008;18:395-399. doi:https://doi.org/10.1055/s-0028-1087221
- Ling S, Sader C, Robbins P. A case of giant ecchordosis physaliphora: a case report and literature review. Otol Neurotol. 2007;28:931-933. doi:https://doi.org/10.1097/mao.0b013e318068b2c8
- Krisht K, Palmer C, Osborn A. Giant ecchordosis physaliphora in an adolescent girl: case report. J Neurosurg Pediatr. 2013;12:328-333. doi:https://doi.org/10.3171/2013.5.peds1395
- Rodríguez L, Colina J, López J. Intradural prepontine growth: giant ecchordosis physaliphora or extraosseous chordoma?. Neuropathology. 1999;19:336-340. doi:https://doi.org/10.1046/j.1440-1789.1999.00241.x
- Ahn S, Han J. Ecchordosis physaliphora presenting with abducens nerve palsy. J AAPOS. 2016;20:266-268. doi:https://doi.org/10.1016/j.jaapos.2016.01.010
- Bolzoni-Villaret A, Stefini R, Fontanella M. Transnasal endoscopic resection of symptomatic ecchordosis physaliphora. Laryngoscope. 2014;124:1325-1328. doi:https://doi.org/10.1002/lary.24434
- Derakhshani A, Livingston S, William C. Spontaneous, intrasphenoidal rupture of ecchordosis physaliphora with pneumocephalus captured during serial imaging and clinical follow-up: pathoanatomic features and management. World Neurosurg. 2020;141:85-90. doi:https://doi.org/10.1016/j.wneu.2020.05.220
- Filis A, Kalakoti P, Nanda A. Symptomatic ecchordosis physaliphora mimicking as an intracranial arachnoid cyst. J Clin Neurosci. 2016;28:171-174. doi:https://doi.org/10.1016/j.jocn.2015.11.018
- Ferguson C, Clarke D, Sinha N. A case study of symptomatic retroclival ecchordosis physaliphora: CT and MR Imaging. Can J Neurol Sci. 2016;43:210-212. doi:https://doi.org/10.1017/cjn.2015.339
- Miki K, Yoshimoto K, Nishimura A. A case of ecchordosis physaliphora in the prepontine cistern: a rare entity in the differential diagnosis of an epidermoid cyst. World Neurosurg. 2017;105:1033.e11-1033.e14. doi:https://doi.org/10.1016/j.wneu.2017.06.003
- Ghimire P, Shapey J, Bodi I. Spontaneous tension pneumocephalus and pneumoventricle in ecchordosis physaliphora: case report of a rare presentation and review of the literature. Br J Neurosurg. 2020;34:537-542. doi:https://doi.org/10.1080/02688697.2019.1594695
- Takeyama J, Hayashi T, Shirane R. Notochordal remnant-derived mass: ecchordosis physaliphora or chordoma?. Pathology. 2006;38:599-600. doi:https://doi.org/10.1080/00313020601023948
- Lagman C, Varshneya K, Sarmiento J. Proposed diagnostic criteria, classification schema, and review of literature of notochord-derived ecchordosis physaliphora. Cureus. 2016;8. doi:https://doi.org/10.7759/cureus.547
- Park H, Lee K, Ahn S. Ecchordosis physaliphora: typical and atypical radiologic features. Neurosurg Rev. 2017;40:87-94. doi:https://doi.org/10.1007/s10143-016-0753-4
- Sooltangos A, Bodi I, Ghimire P. Do all notochordal lesions require proton beam radiotherapy? A proposed reclassification of ecchordosis physaliphora as benign notochord cell tumor. J Neurol Surg B Skull Base. 2021;83:E96-E104. doi:https://doi.org/10.1055/s-0040-1722717
- Chihara C, Korogi Y, Kakeda S. Ecchordosis physaliphora and its variants: proposed new classification based on high-resolution fast MR imaging employing steady-state acquisition. Eur Radiol. 2013;23:2854-2860. doi:https://doi.org/10.1007/s00330-013-2888-9
- Toda H, Kondo A, Iwasaki K. Neuroradiological characteristics of ecchordosis physaliphora. Case report and review of the literature. J Neurosurg. 1998;89:830-834. doi:https://doi.org/10.3171/jns.1998.89.5.0830
- Stam F, Kamphorst W. Ecchordosis physaliphora as a cause of fatal pontine hemorrhage. Eur Neurol. 1982;21:90-93. doi:https://doi.org/10.1159/000115460
- Fracasso T, Brinkmann B, Paulus W. Sudden death due to subarachnoid bleeding from ecchordosis physaliphora. Int J Legal Med. 2008;122:225-227. doi:https://doi.org/10.1007/s00414-007-0192-4
- Georgalas C, Terzakis D, Tsikna M. Ecchordosis physaliphora: a cautionary tale. J Laryngol Otol. 2020;134:46-51. doi:https://doi.org/10.1017/s0022215119002512
- Galloway L, Hayhurst C. Spontaneous cerebrospinal fluid rhinorrhoea with meningitis secondary to ecchordosis physaliphora. Br J Neurosurg. 2019;33:99-100. doi:https://doi.org/10.1080/02688697.2017.1297766
- Sun R, Ajam Y, Campbell G. A rare case of ecchordosis physaliphora presenting with headache, abducens nerve palsy, and intracranial hypertension. Cureus. 2020;12. doi:https://doi.org/10.7759/cureus.8843
- Castello Ruiz M, Alsavaf M, Fadel M. Spontaneous rhinorrhea: a possible concealing initial symptom of ecchordosis physaliphora. Illustrative case. J Neurosurg Case Lessons. 2023;5. doi:https://doi.org/10.3171/case236
- Zhong X, Huang B, Liu C. Multiple ecchordosis physaliphora: a challenging diagnosis. Chin Med J (Engl). 2015;128:2826-2828. doi:https://doi.org/10.4103/0366-6999.167368
- Yamamoto T, Yano S, Hide T. A case of ecchordosis physaliphora presenting with an abducens nerve palsy: a rare symptomatic case managed with endoscopic endonasal transsphenoidal surgery. Surg Neurol Int. 2013;4. doi:https://doi.org/10.4103/2152-7806.106562
- Macdonald R, Cusimano M, Deck J. Cerebrospinal fluid fistula secondary to ecchordosis physaliphora. Neurosurgery. 1990;26:515-519. doi:https://doi.org/10.1097/00006123-199003000-00022
- Watanabe A, Yanagita M, Ishii R. Magnetic resonance imaging of ecchordosis physaliphora − Case report. Neurol Med Chir (Tokyo). 1994;34:448-450. doi:https://doi.org/10.2176/nmc.34.448
- Akimoto J, Takeda H, Hashimoto T. [A surgical case of ecchordosis physaliphora]. No Shinkei Geka. 1996;24:1021-1025.
- Cha S, Jarrahy R, Yong W. A rare symptomatic presentation of ecchordosis physaliphora and unique endoscope-assisted surgical management. Minim Invasive Neurosurg. 2002;45:36-40. doi:https://doi.org/10.1055/s-2002-23584
- Rotondo M, Natale M, Mirone G. A rare symptomatic presentation of ecchordosis physaliphora: neuroradiological and surgical management. J Neurol Neurosurg Psychiatry. 2007;78:647-649. doi:https://doi.org/10.1136/jnnp.2006.109561
- Adib S, Bisdas S, Bornemann A. Neuroendoscopic trans-third ventricular approach for surgical management of ecchordosis physaliphora. World Neurosurg. 2016;90:701.e1-701.e6. doi:https://doi.org/10.1016/j.wneu.2016.02.041
- Raffa A. Atypical presentation and neuroradiological features of giant ecchordosis physalyphora in a seven-year-old patient: a case report. Cureus. 2022;14. doi:https://doi.org/10.7759/cureus.23544
- Kaul S, Khan O, Edem I. Transclival pseudomeningocele secondary to ecchordosis physaliphora: case report and literature review. J Neurol Surg Rep. 2013;74:92-95. doi:https://doi.org/10.1055/s-0033-1348956
- Gupta R, Reddy T, Gupta A. An ecchordosis physaliphora, a rare entity, involving the central nervous system: a systematic review of the literature. Neurol Int. 2023;15:1200-1211. doi:https://doi.org/10.3390/neurolint15040075
- Hasegawa H, Van Gompel J, Choby G. Unrecognized notochordal lesions as a likely cause of idiopathic clival cerebrospinal fluid leaks. Clin Neurol Neurosurg. 2023;224. doi:https://doi.org/10.1016/j.clineuro.2022.107562
- Stuebe C, Rindler R, Laack N. Evaluation of long-term follow-up in ecchordosis physaliphora versus chordoma. World Neurosurg. 2023;174:157-168. doi:https://doi.org/10.1016/j.wneu.2023.03.016
- Ang L, Kew T, Toh C. Ecchordosis physaliphora masquerading as chordoma: a case report. Hong Kong J Radiol. 2020;23:223-226. doi:https://doi.org/10.12809/hkjr2017111
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 799 times
- PDF downloaded - 127 times



