Laryngology
Vol. 44: Issue 4 - August 2024
Recurrent respiratory papillomatosis: comparing in-office and operating room treatments
Abstract
Objective. We report the management of recurrent respiratory papillomatosis (RRP) employing a protocol that includes both office-based (OB) and general anaesthesia (GA) procedures. Quality of life (QoL) outcomes in the OB cohort were compared to those obtained from an historical cohort treated only under GA.
Methods. Patients affected by RRP from 2019 until 2023 (“new protocol”) and from 2012 to 2019 (“historical protocol”) were enrolled. In both groups the Derkay site score (DSS) was calculated. In patients adhering to the new protocol, questionnaires measuring QoL were prospectively administered (voice handicap hindex-10 [VHI-10] along with a specific questionnaire to measure the tolerance to the OB procedures). A cost analysis was also performed.
Results. In all, 35 patients composed the new protocol cohort and 13 the historical. In the first group, patients underwent a median of 4 treatments. At 2 years, 68% of patients were treated exclusively in the office. Overall, for the new protocol, median DSS and VHI-10 after one year were both significantly lower than those at baseline [2 vs 4 and 3 vs 14, respectively; p < 0.001]. No differences were found between the new and the historical protocol cohorts considering DSS over time.
Conclusions. Treatment of RRP may be conducted successfully in an office-based setting reducing healthcare costs.
Introduction
Recurrent respiratory papillomatosis (RRP) is a relatively rare disease that affects the upper aerodigestive tract, predominantly the larynx. Human papillomavirus (HPV) 6 and 11 are responsible for more than 90% of cases of RRP 1. HPV 16 and 18 are high-risk subtypes, with the potential for malignant transformation, and are present in less than 1% of RRP cases. Patients presenting with this disease after 12 years of age are diagnosed with adult-onset recurrent respiratory papillomatosis (Ao-RRP). The incidence of Ao-RRP has been reported between 3 and 10 per 1,000,000, while it most commonly presents between 20 and 40 years of age, with a higher prevalence in men 2.
Currently, there is no curative treatment for RRP, and several therapeutic strategies have been proposed with the main goals of keeping the airway patent and maintaining a satisfactory voice quality. Surgery still represents the standard of care, while there is no solid evidence for proposing a standardised adjuvant protocol 3. In this light, patients frequently require several surgeries in a short time frame in order to control the disease with frequent hospitalisations. It has been reported that the mean number of surgeries in the first 5 years after diagnosis is 5.1 per year, decreasing to 0.1 per year after 15 years 4. Traditional management of RRP has been surgical excision in the operating room (OR) under general anaesthesia (GA) with carbon dioxide (CO2) laser or potassium-titanyl-phosphate (KTP) laser, microdebriders, or, rarely, cold steel instrumentation. On the other hand, the advent of high-definition flexible videoendoscopes with a working channel has made laser procedures available in-office using glass fibre lasers such as Trueblue (TBL) or KTP. This therapeutic option is showing emerging interest in the scientific community as an alternative to traditional procedures under GA. Moreover, it offers a valuable cost-effectiveness ratio in terms of healthcare costs and need for hospitalisation 5. The aim of this retrospective study was to analyse the trends of disease control and voice-related quality of life during follow-up (FU) in a cohort of patients affected by Ao-RRP who underwent office-based (OB) laser procedures. Moreover, we compared the outcomes of this treatment strategy with those achieved in a historical group of patients treated under GA in the OR.
Materials and methods
A prospective study was carried out by enrolling adult patients affected by RRP after the introduction of an in-office laryngeal procedure in September 2019 until September 2023 at the Unit of Otolaryngology-Head and Neck Surgery of IRCCS Policlinico San Martino Hospital - University of Genoa. Inclusion criteria were: (1) age >18 years; (2) biopsy-proven RRP. Exclusion criteria were: (1) patients who dropped out of FU after the first treatment.
The protocol applied in this group of patients (called “new protocol”) consisted of treatment by transoral laser surgery (TOLMS) under GA and/or transnasal flexible fibreoptic treatment in the office under local anaesthesia (OB) 6. Patients with no histopathologic diagnosis underwent a prior biopsy under GA or in the office. After the first treatment, an endoscopic FU was planned every 2-3 months. If disease recurrence was detected, the patient was submitted to treatment. An OB procedure was proposed if the endoscopic examination was well tolerated, if the disease was not too extended, and if no breathing difficulty was present. If a patient was disease-free for 2 clinical evaluations in a row, the following FU was scheduled after 6 months. At each FU, disease extension and the quality of voice were recorded prior to treatment. Intramuscular injections of three doses of Gardasil 9® (HPV 9-valent vaccine) were recommended to patients who had not already been vaccinated.
The charts from an historical cohort of patients from September 2012 to September 2019 were retrospectively collected in order to compare outcomes in the two groups. The same inclusion and exclusion criteria were used together with the availability of the recorded endoscopies at the moment of FU. The treatment protocol applied in this cohort of patients (called “historical protocol”) consisted in TOLMS procedures under GA. Conversely from the OB treatment group, these patients were followed and not treated according to a fixed schedule, but based on disease presentation and patient’s symptoms. As the enrollment of the historical group was retrospective, only Derkay site score (DSS) was retrievable and therefore the two treatment groups were compared based on this outcome.
The endoscopic evaluation through a transnasal route was performed with a flexible high-definition videoendoscope (Olympus ENF-VT3, Olympus Medical System Corporation, Tokyo, Japan), both in white light (WL) and narrow band imaging (NBI), connected to a Visera Elite CLV-S190 light source (Olympus Medical System Corporation). The DSS was calculated at the time of each FU based on the endoscopic examination prior to the procedure according to the Derkay staging system 7 (Tab. I).
Transoral laser microsurgery
When the treatment was performed by TOLMS, the patient was intubated with a 5-6 mm internal diameter orotracheal tube (Shiley™ Laser Oral Endotracheal Tubes, Medtronic Xomed, Jacksonville, FL, USA). In cases of bulky papillomas involving the posterior commissure, subglottic area, or extending to the trachea, a narrow-cuffed ventilation catheter (Evone®; Ventinova, Eindhoven, Netherlands) was used. High-frequency jet ventilation was not utilised to avoid the risk of dissemination of papillomas and aerosolisation of viral particles for the OR staff 8. CO2 laser or TBL were preferred to treat the papillomatous lesions, as both provide precise excision and accurate haemostasis, while a microdebrider was used only in selected cases for debulking obstructing lesions in combination with lasers.
Office-based procedures
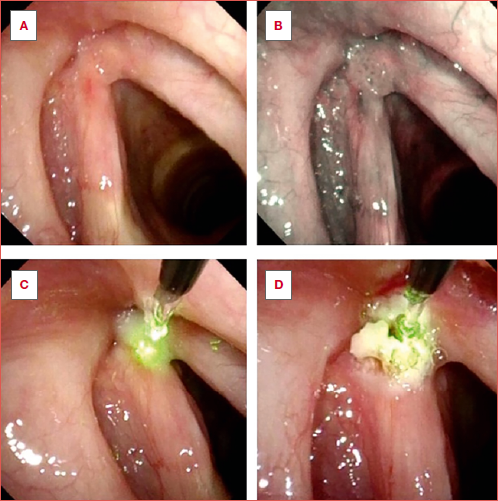
Patients were treated in the office if they demonstrated good compliance during a prior flexible laryngoscopy. Those who did not tolerate the procedure, or refused to undergo the treatment under local anaesthesia, were managed under GA. Very advanced RRP (DSS > 6) or causing significant airway obstruction were also treated in the OR. OB procedures were performed using a flexible high-definition videoendoscope with a working operative channel (Olympus ENF-VT3, Olympus Medical System Corporation, Tokyo, Japan). A standardised protocol was applied to optimize the patient’s compliance. Firstly, 2-3 ml of 5% neobucaine was administered transnasally, and an additional 3-4 ml of neobucaine 5% was sprayed directly above the glottic plane through the operative channel 9. A 445 nm diode blue laser (TruBlue, A.R.C. Laser Company, Nuremberg, Germany) with a 400 μm fibre diameter was used during all OB procedures with the following settings: 10 W, 30 msec pulses, 150 msec pauses. Superficial papillomas were treated by photocoagulation to obtain a superficial blanching of their surface and afferent vessels. In case of exophytic lesions, the laser tip was inserted into the papillomas to obtain their shrinkage; during this latter step, special care was paid to avoid any contact and thermal damage to the underlying vocal ligament (Cover Figure). Selected patients affected by massive bilateral/commissural vocal fold RRP were managed with both modalities using a staged procedure (first TOLMS and after OB) to reduce the risk of iatrogenic sequelae such as synechiae or more complex stenosis.
Questionnaries
To evaluate voice-related QoL, the Voice Handicap Index-10 (VHI-10) was administered to patients at every FU before possible treatment 10. The VHI-10 consists of 10 items distributed over three domains of voice disorders: functional (5 items), physical (3 items), and emotional (2 items). Patients subjectively answered each question using a 5-point scale ranging from 0 (never) to 4 (always).
To assess tolerance to OB procedures a specific questionnaire was administrated immediately after the first OB treatment. Patients were asked to rate “overall pain”, “nose pain”, “throat pain” and “after procedure pain” on a scale from 1 to 10 (with 1 being intolerable and 10 being no pain). In addition, a discomfort scale (0 = no discomfort, 100 = maximal discomfort) was used to assess the overall unpleasantness of the intervention.
Cost assessment
A cost assessment was drawn up by comparing TOLMS and OB costs for a single RRP procedure. The costs of the TOLMS included preoperative outpatient clinic assessment, supplies, OR and GA costs, recovery room, one-night hospitalisation (as for our hospital protocol), and staff fees. For OB procedures, the costs of outpatient clinic examination, local anesthetic drugs, fiber laser supply, instrument sterilisation, and staff fees were considered.
Statistical analysis
Patient age, gender, smoking habit, number of previous treatments, Derkay scores, VHI-10, tolerance scores, number of treatments, and FU time were analysed and summarised as median and interquartile range (IQR) and frequencies as appropriate. Differences in the distribution of categorical variables between groups were tested by χ2 or Fisher’s exact test. Differences among continuous variables between groups were tested using the Mann-Whitney U test. The differences among the scores at each FU time within the same protocol group were compared using the Friednman test: in case of significant differences, post-hoc multiple comparisons using Wilcoxon’s test were performed adjusting according to Bonferroni’s method to control for the inflated Type I error. Differences between the two groups at each FU time point were analysed with the Mann Whitney U test and applying the Bonferroni correction. A p-value < 0.05 was considered significant if not corrected. Data analysis was carried out using R software for statistical computing version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Forty-eight patients met the inclusion criteria and were prospectively enrolled. Thirteen underwent the historical protocol and 35 were enrolled in the new one. Table II overviews the demographics and type and number of treatments for each group. The two cohorts were homogeneous in terms of age (p = 0.99), gender (p = 0.99), smoking history, number of previous treatments (p = 0.52) and FU time (p = 0.15).
In the new protocol cohort, a total of 190 procedures were carried out: 160 (84%) OB and 30 (16%) in the OR. Patients were followed during a median period of 26 (range 5-77) months. Considering both OB and OR surgeries, each patient underwent a median of 4 (range 1-17) treatments corresponding to almost 2 sessions per year. No major complications occurred during outpatient treatments. Two patients had an episode of laryngospasm during the procedure, but no treatment was required since, by interrupting the procedure, both patients recovered spontaneously.
Twenty-five patients answered the procedure discomfort assessment questionnaire. The median (IQR) results were the following: overall pain = 8(5); nose pain = 9(3); throat pain = 8(5); after-procedure pain = 9(4); overall discomfort scale = 40(50). Among the 30 treatments conducted under GA, only 16 were required by 11 patients after the first standardised GA baseline treatment at our centre. Among these, only 2 patients did not tolerate the procedure in the office under local anaesthesia and refused to undergo the OB treatment, and thus were treated by TOLMS under GA.
Figure 1 shows the Kaplan-Meier curve estimating the risk for the new protocol cohort of undergoing a GA procedure over time. At 2 years, 68% of patients had been treated exclusively in the office. Figures 2 and 3 show the boxplots representing the trends of DSS and VHI-10 during the first 4 FUs, roughly corresponding to 1 year of FU. Overall, the median DSS, and VHI-10 at the fourth FU were all significantly lower compared to the first assessment at presentation [2(6) vs 4(4), p < 0.001; 3(11) vs 14(17), p < 0.001, respectively]. A total of 12 (36%) patients were disease-free at the time of the last endoscopic examination. No patient underwent tracheotomy during FU, while one patient who was previously tracheotomised in another centre was decannulated.
Figure 4 shows disease control measured by DSS over time in the old (n = 13) and new (n = 35) treatment cohorts. As reported in Table III, no differences were found between the two approaches at any FU time point.
Among all the procedures, we registered one case of laryngospasm and one of vagal reaction, classified as grade I according to the Clavien-Dindo classification 11.
Finally, the OB and OR treatments costs were estimated to be approximately €750 and €2142, respectively. Since during 4 years, a total of 136 OB treatments were carried out, the approximate savings were around €189,300 (Tab. IV).
Discussion
Nowadays, there are no definitive treatments able to eradicate RRP, which is characterised by continuous disease relapses that require repeated procedures. On average, a patient affected by RRP will require 4.4 interventions per year, while those with severe disease may necessitate surgery every 4-6 weeks to maintain a clear airway 12,13. In our cohort, there was a mean of 2.8 treatments per year (either under GA or OB), which is slightly less than that reported in the literature, likely because all patients were adults who generally have a better disease course than children.
Historically, TOLMS has been the mainstay of treatment for RRP, but its use comes with an important discomfort for the patient and high healthcare costs. Moreover, its timely use is limited by the necessity of an OR and the patient’s reluctance to frequently undergo this procedure 14. To overcome the risks and costs of GA, OB procedures are becoming increasingly popular (Tab. IV).
High-definition channeled endoscopes with distal chip technology and improved laser mechanics have all prompted the exploration of OB surgery for RRP. The reduced social burden for patients who undergo in-office surgeries leads to earlier and more frequent procedures and shifts the treatment goal to not just maintaining airway patency, but allowing a more consistent and constant voice quality and general QoL 15,16. This idea was pioneered and introduced as a management option for RRP by Zeitels et al. in 2004, who demonstrated that the pulsed-dye laser could safely and successfully be used in an OB setting for the treatment of RRP 15. In his cohort, 93.9% of patients tolerated the procedure, similar to our data where 31 of 33 (94%) felt comfortable with the in-office procedures. While the KTP laser has been the most utilised glass fibre laser in the OB setting to treat various laryngeal diseases 14,17, the TBL has recently been proposed to treat benign neoplasms arising from the larynx 18,19. The reported advantages of this device include portability and a desirable blend of photoangiolytic and cutting properties 18,20. Furthermore, recent animal-based studies have demonstrated reduced postoperative fibrosis and scarring with TBL compared with KTP 21, although there are still no prospective in vivo studies comparing these two lasers.
It is important to stress that the first goal of RRP treatment remains to maintain airway patency and to avoid iatrogenic sequelae. Indeed, since RRP patients will probably need a significant number of surgeries in their lifetime, avoiding synechia formation is of paramount importance. The application of our new protocol allowed us to avoid this insidious complication since patients characterised by a high disease burden (especially when extended to the glottic and anterior commissure region) were treated by combining procedures under GA and OB. First, the disease was treated in a single stage procedure under GA, leaving a small disease remnant in the anterior and/or posterior commissural area; lastly, after the complete healing process, the same remnant was cleared in the outpatient setting with a TBL procedure.
Moreover, tracheotomies should be limited to extremely selected emergency cases, since they may result in rapid colonisation and distal spread of the disease 22. In our cohort, this goal was achieved by following patients closely and treating even small papillomas in the office to prevent them from overgrowing. The only patient who required emergency treatment due to respiratory distress was successfully managed without a tracheotomy with an Evone tube 8.
The second goal of treatment is to maintain a good quality of voice over time. In our cohort, the mean VHI-10 score before treatment (14.9 ± 10) was significantly higher (p < 0.001) than that at the last FU visit (5.7 ± 8.5) and this value did not change significantly during FU.
OB procedures have already been demonstrated to be comparable to GA treatments in terms of vocal outcomes for benign laryngeal neoplasms 19, despite the fact that regularly treating a patient in the office will lead to more treatments than in the OR. Nonetheless, it has been demonstrated that the overall number of surgeries did not significantly affect mean postoperative voice outcomes over time 23. This can be explained considering that the disease threshold for OB intervention is considerably lower, and patients can be treated for disease severity who would otherwise be deferred, leading to a more stable voice. Considering the subgroup of patients who followed the historical protocol, our results showed an improvement in DSS measured after the first treatment that remained stable over time.
Overall, these findings confirm the importance of careful and close FU of patients with RRP, which leads to a reduced risk of emergency surgery and consistently good voice quality. Furthermore, this corroborates our belief that our new protocol for these complex patients can provide them a better overall QoL and disease control.
Regarding costs, we observed significant savings by performing OB treatments, however hospital remuneration for outpatient procedures is not yet standardised (Tab. IV). Similarly, Schimberg et al. revealed that laryngopharyngeal treatments under topical anaesthesia resulted in hospital cost reductions of up to 95% per procedure compared to similar procedures under GA, even though nowadays they are not adequately reimbursed 24.
Concerning the safety of OB procedures, there is a lack of reports regarding long-term experiences even though the existing data from the literature indicate that, in experienced hands, they are safe and reliable 24. Woisard et al. recently reported grade I and II complications in 9.1% and 1.2% of patients respectively, according to the Clavien-Dindo classification system 25. Similarly, in our cohort we did not experience any major complications, but only 2 grade I events. Nevertheless, considering the absence of anesthesiology professionals involved in the procedures, we recommend the availability of oxygen and an emergency trolley equipped with a defibrillator in the area.
In the present prospective study, we report our experience in treating Ao-RRP proposing a close FU protocol and a combination of OB and GA treatments to better manage this challenging disease. Indeed, this insidious pathology diversifies the course and prognosis of each patient, thus making it complicated to define a general management protocol. In our experience, establishing close FU and promptly performing procedures in the office, when possible, allowed us to provide patients with a tailored management protocol that led to very satisfying and stable outcomes. Nevertheless, several limitations should be acknowledged. First, the short and inconsistent FU represents an inherent bias. Moreover, it was not possible to have a diagnosis of most HPV subtypes, which made it impossible to consider these findings for statistical analysis. Finally, an obvious limit is the small study population. Future investigations on larger cohorts in a randomised and multicentric fashion are needed to identify the best FU and treatment protocols for such a challenging disease.
Conclusions
RRP represents a challenging and often unremitting disease that requires multiple procedures to maintain airway patency and voice quality. Surgery is the primary treatment strategy and recent advances in flexible laryngoscopy and laser technology have allowed for an increase in the use of OB procedures. Our experience confirms that the surgical care of RRP may now be conducted successfully in the office. This treatment strategy demonstrated to be safe in experienced hands, but long-term reports on safety are lacking. Nevertheless, this new treatment paradigm is particularly appealing as, while saving time for both the patient and the medical professionals, it can significantly reduce healthcare costs. A close FU for early detection and treatment of limited disease can lead to a stably lower burden of disease and consistently better voice-related QoL over time.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
MF, FM, AV: designed the study, performed literature search and contribute to paper revision; CS, AI: analysed the data and contributed to paper revision. PB, GG, MDV collected the data; GP: contributed to paper revision. All the authors read and approved the final version of the manuscript.
Ethical consideration
This study was approved by the Institutional Ethics Committee (Comitato Etico Regionale della Liguria, protocol number 63/2021).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from each patient for study participation and data publication.
History
Received: February 6, 2024
Accepted: April 13, 2024
Figures and tables
Figure 1. Kaplan-Meier curve estimates the risk for the new treatment protocol cohort of undergoing a general anaesthesia procedure throughout the time.
Figure 2. Boxplots of the recorded Derkay Site Scores at sequential time points during the first year of follow-up for the new protocol.
Figure 3. Boxplots of the 10-item Voice Handicap Index scores at sequential time points during the first year of follow-up for the new protocol.
Figure 4. Boxplots representing the Derkay Site Scores for each treatment at sequential time points during the first year of follow-up after the first treatment.
| Right | Left | ||
|---|---|---|---|
| Larynx | Epiglottis | ||
| Aryepiglottic folds | |||
| False vocal cords | |||
| True vocal cords | |||
| Arytenoids | |||
| Anterior commissure | |||
| Posterior commissure | |||
| Subglottis | |||
| Trachea | Upper one-third | ||
| Middle one-third | |||
| Lower one-third | |||
| Bronchi | |||
| Tracheostomy | |||
| Other | Nose | ||
| Palate | |||
| Pharynx | |||
| Oesophagus | |||
| Lungs | |||
| Other | |||
| Total score all sites: ____ | |||
| For each site, score as 0= none, 1= surface lesion, 2= raised lesion, 3= bulky lesion. | |||
| Variable | Overall (n = 48) | Treatment protocol | p value | |
|---|---|---|---|---|
| New (n = 35) | Historical (n = 13) | |||
| Age (years) | 0.18 | |||
| Median (IQR) | 45 (26.7) | 46 (27.5) | 40(21) | |
| Range | 14-83 | 19-83 | 14-78 | |
| Gender | > 0.99 | |||
| Female | 17 (35%) | 12 (34%) | 5 (38%) | |
| Male | 31 (65%) | 23 (66%) | 8 (62%) | |
| Smoking | > 0.99 | |||
| No | 33 (82%) | 27 (82%) | 6 (86%) | |
| Yes | 7 (18%) | 6 (18%) | 1 (14%) | |
| No. of previous treatments | 0.15 | |||
| Median (IQR) | 2(2) | 2 (3.5) | 1(2) | |
| Range | 0-29 | 0-29 | 0-7 | |
| FU time (months) | 0.52 | |||
| Median (IQR) | 29 (30.2) | 26 (22.5) | 36(54) | |
| Range | 1-83 | 5-77 | 1-83 | |
| No. of treatments | 0.42 | |||
| Median (IQR) | 4(4) | 4(4) | 4(3) | |
| Range | 1-17 | 1-17 | 2-15 | |
| No. office-based treatments | < 0.001 | |||
| Median (IQR) | 3 (5.2) | 3 (3.5) | 0 (0) | |
| Range | 0-17 | 0-17 | 0-0 | |
| No. general anaesthesia treatments | < 0.001 | |||
| Median (IQR) | 1 (2.2) | 1(1) | 4(3) | |
| Range | 0-15 | 0-4 | 2-15 | |
| No. of no-treatments | < 0.001 | |||
| Median (IQR) | 0(2) | 1(2) | 0 (0) | |
| Range | 0-7 | 0-7 | 0-0 | |
| Time interval between procedures (months) | 0.26 | |||
| Median (IQR) | 3.45 (6.1) | 3.22 (3.9) | 8.4(9) | |
| Range | 1-64 | 1-14.2 | 1-64 | |
| IQR: interquartile range. FU: follow-up. Differences between the two treatment groups were analysed with the Mann Whitney U and χ2 or Fisher’s exact test when appropriate. | ||||
| Follow-up Derkay Site Score | ||||
|---|---|---|---|---|
| Variable | Overall (n = 65) | Protocol | p value | |
| New (n = 35) | Historical (n = 13) | |||
| Baseline Derkay site score | 0.47 | |||
| Median (IQR) | 4(3) | 4 (3.5) | 4(3) | |
| Range | 0-13 | 0-13 | 3-8 | |
| 1st FU Derkay site score | 0.34 | |||
| Median (IQR) | 2.5(4) | 2(3) | 3(4) | |
| Range | 0-10 | 0-8 | 1-10 | |
| 2nd FU Derkay site score | 0.09 | |||
| Median (IQR) | 2(3) | 2 (2.2) | 3(2) | |
| Range | 0-6 | 0-6 | 1-6 | |
| 3rd FU Derkay site score | 0.24 | |||
| Median (IQR) | 2(3) | 2 (3.5) | 3.5 (3.7) | |
| Range | 0-10 | 0-10 | 0-7 | |
| 4th FU Derkay site score | 0.29 | |||
| Median (IQR) | 2(5) | 2(5) | 4 (1.5) | |
| Range | 0-8 | 0-8 | 0-7 | |
| IQR: interquartile range. Differences between the two treatment groups at each follow-up (FU) time point were analysed with the Mann Whitney U test. | ||||
| Procedures | Cost in Euros (€) |
|---|---|
| Benign microlaryngoscopy in the operating room | 2142 |
| Laryngeal procedures, office-based | 750 |
References
- Derkay C, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236-1247. doi:https://doi.org/10.1097/MLG.0b013e31816a7135
- Taliercio S, Cespedes M, Born H. Adult-onset recurrent respiratory papillomatosis: a review of disease pathogenesis and implications for patient counseling. JAMA Otolaryngol Head Neck Surg. 2015;141:78-83. doi:https://doi.org/10.1001/jamaoto.2014.2826
- Bertino G, Pedretti F, Mauramati S. Recurrent laryngeal papillomatosis: multimodal therapeutic strategies. Literature review and multicentre retrospective study. Acta Otorhinolaryngol Ital. 2023;43:111-122. doi:https://doi.org/10.14639/0392-100X-suppl.1-43-2023-14
- Silverberg M, Thorsen P, Lindeberg H. Clinical course of recurrent respiratory papillomatosis in Danish children. Arch Otolaryngol Head Neck Surg. 2004;130:711-716. doi:https://doi.org/10.1001/archotol.130.6.711
- Shah M, Johns M. Office-based laryngeal procedures. Otolaryngol Clin North Am. 2013;46:75-84. doi:https://doi.org/10.1016/j.otc.2012.08.019
- Remacle M, Arens C, Eldin M. Laser-assisted surgery of the upper aero-digestive tract: a clarification of nomenclature. A consensus statement of the European Laryngological Society. Eur Arch Otorhinolaryngol. 2017;274:3723-3727. doi:https://doi.org/10.1007/s00405-017-4708-3
- Derkay C, Malis D, Zalzal G. A staging system for assessing severity of disease and response to therapy in recurrent respiratory papillomatosis. Laryngoscope. 1998;108:935-937. doi:https://doi.org/10.1097/00005537-199806000-00026
- Filauro M, Mora F, Vallin A. Evone® Flow controlled ventilation: a new device for laryngotracheal surgery. Acta Otorhinolaryngol Ital. 2022;42:189-193. doi:https://doi.org/10.14639/0392-100X-N1834
- Filauro M, Vallin A, Fragale M. Office-based procedures in laryngology. Acta Otorhinolaryngol Ital. 2021;41:243-247. doi:https://doi.org/10.14639/0392-100X-N0935
- Jacobson B, Johnson A, Grywalski C. The Voice Handicap Index (VHI): development and validation. Am J Speech Lang Pathol. 1997;6:66-69. doi:https://doi.org/10.1044/1058-0360.0603.66
- Clavien P, Barkun J, de Oliveira M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. doi:https://doi.org/10.1097/SLA.0b013e3181b13ca2
- Armstrong L, Derkay C, Reeves W. Initial results from the national registry for juvenile-onset recurrent respiratory papillomatosis. RRP Task Force. Arch Otolaryngol Head Neck Surg. 1999;125:743-748. doi:https://doi.org/10.1001/archotol.125.7.743
- Bishai D, Kashima H, Shah K. The cost of juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2000;126:935-939. doi:https://doi.org/10.1001/archotol.126.8.935
- Motz K, Hillel A. Office-based management of recurrent respiratory papilloma. Curr Otorhinolaryngol Rep. 2016;4:90-98. doi:https://doi.org/10.1007/s40136-016-0118-0
- Zeitels S, Franco R, Dailey S. Office-based treatment of glottal dysplasia and papillomatosis with the 585-nm pulsed dye laser and local anesthesia. Ann Otol Rhinol Laryngol. 2004;113:265-276. doi:https://doi.org/10.1177/000348940411300403
- Rees C, Halum S, Wijewickrama R. Patient tolerance of in-office pulsed dye laser treatments to the upper aerodigestive tract. Otolaryngol Head Neck Surg. 2006;134:1023-1027. doi:https://doi.org/10.1016/j.otohns.2006.01.019
- Zeitels S, Akst L, Burns J. Office-based 532-nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann Otol Rhinol Laryngol. 2006;115:679-685. doi:https://doi.org/10.1177/000348940611500905
- Hess M, Fleischer S, Ernstberger M. New 445 nm blue laser for laryngeal surgery combines photoangiolytic and cutting properties. Eur Arch Otorhinolaryngol. 2018;275:1557-1567. doi:https://doi.org/10.1007/s00405-018-4974-8
- Filauro M, Ioppi A, Vallin A. Office-based treatment of vocal fold polyps and Reinke’s edema: a rational comparison with suspension laryngoscopy. Laryngoscope. 2023;133:2665-2672. doi:https://doi.org/10.1002/lary.30576
- Miller B, Abdelhamid A, Karagama Y. Applications of office-based 445 nm blue laser transnasal flexible laser surgery: a case series and review of practice. Ear Nose Throat J. 2021;100:105S-112S. doi:https://doi.org/10.1177/0145561320960544
- Lin R, Iakovlev V, Streutker C. Blue light laser results in less vocal fold scarring compared to KTP laser in normal rat vocal folds. Laryngoscope. 2021;131:853-858. doi:https://doi.org/10.1002/lary.28892
- Cole R, Myer C, Cotton R. Tracheotomy in children with recurrent respiratory papillomatosis. Head Neck. 1989;11:226-230. doi:https://doi.org/10.1002/hed.2880110306
- Parker L, Kunduk M, Blouin D. Voice outcomes following multiple surgeries for recurrent respiratory papillomatosis. J Voice. 2020;34:791-798. doi:https://doi.org/10.1016/j.jvoice.2019.02.004
- Schimberg A, Wellenstein D, van den Broek E. Office-based vs. operating room-performed laryngopharyngeal surgery: a review of cost differences. Eur Arch Otorhinolaryngol. 2019;276:2963-2973. doi:https://doi.org/10.1007/s00405-019-05617-z
- Woisard V, Alexis M, Crestani S. Safety of office-based flexible endoscopic procedures of the pharynx and larynx under topical anesthesia. Eur Arch Otorhinolaryngol. 2022;279:5939-5943. doi:https://doi.org/10.1007/s00405-022-07525-1
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2024 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1319 times
- PDF downloaded - 294 times




