Vestibology
Vol. 45: Issue 4 - August 2025
Impact of haemodialysis on vestibular function in adult patients with chronic kidney disease
Haemodialysis and vestibular function
Abstract
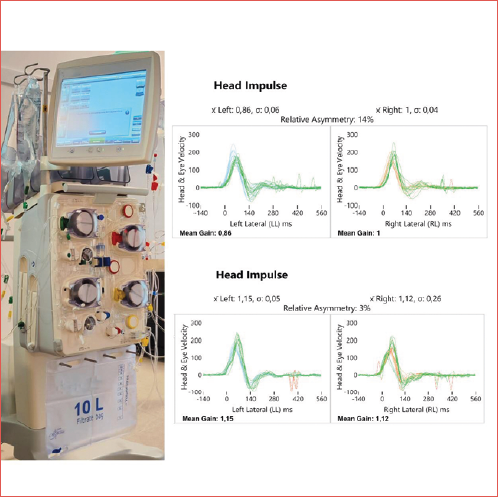
Objective. Chronic kidney disease (CKD) is a global health problem with a significant impact on patients’ quality of life. Haemodialysis, a common treatment for advanced CKD, can profoundly affect vestibular function, which plays a critical role in maintaining balance and spatial orientation.
Methods. This prospective study was conducted over a 6-month period at the Magna Graecia University and included 18 adult patients with CKD stage 5, undergoing haemodialysis. Vestibular function was assessed using the Visual Analogue Scale (VAS) for the symptom of unsteadiness and the video Head Impulse Test (vHIT) for the vestibulo-ocular reflex (VOR) gain.
Results. Our results showed a statistically significant decrease in VOR gain from 0.99 at T0 to 0.92 at T1 (T-test p = 0.034 and Welch Test p = 0.037), accompanied by an increase in VAS instability scores, after the dialysis session. These results suggest a worsening of vestibular function as a result of haemodialysis.
Conclusions. These results highlight the need for early diagnosis and timely intervention, such as vestibular rehabilitation, to reduce the risk of falls and improve the quality of life in CKD patients undergoing haemodialysis. Future research should investigate the long-term effects of haemodialysis on vestibular function.
Introduction
Chronic kidney disease (CKD) is a major global health problem affecting millions of people worldwide. It is a clinical syndrome resulting from a definitive change in renal function and/or structure, characterised by irreversibility and a slow, progressive course. In its advanced stages, CKD is associated with an accumulation of fluids, electrolytes and wastes in the body, potentially necessitating dialysis or kidney transplantation. Haemodialysis, or extrarenal dialysis, is a therapy that replaces kidney function and is given to people with critically reduced kidney function in the most severe stage of kidney failure 1. The primary goals of haemodialysis are to correct hydroelectrolyte imbalances and remove toxins 2,3. It can also improve the prognosis of patients with chronic kidney failure by improving arterial pressure and preventing complications from accumulation of urea, which can be extremely life-threatening 4. The impact of CKD and haemodialysis on a patient’s quality of life is profound. The physical and emotional burden of managing a chronic disease, coupled with the time-consuming and often exhausting process of haemodialysis, can significantly affect a patient’s overall well-being 5. In addition, the potential complications and side effects associated with haemodialysis may exacerbate the challenges faced by these patients.
In particular, CKD and haemodialysis can have a direct impact on vestibular function 6,7, although the involvement of the vestibular system in patients with renal failure has not been studied as much as the involvement of the cochlea and hearing.
The vestibular system, which plays a crucial role in maintaining balance and spatial orientation, can be affected by the changes in fluid and electrolyte balance that occur in CKD and during haemodialysis 8. These changes can lead to vestibular dysfunction, manifesting such as symptoms of dizziness, imbalance and vertigo, which can significantly impair a patient’s ability to perform daily activities and reduce their overall quality of life 9.
In a study by Varghese and colleagues 10, abnormal ocular and cervical vestibular evoked myogenic potential (oVEMP and cVEMPS) findings in individuals with CKD treated with haemodialysis suggested the presence of otolithic dysfunction, and their amplitude was found to decrease with an increase in the disease duration.
Moreover, Ozmen et al. 11 in a recent study investigated possible modifications in vestibular system in adult CDK patients treated with haemodialysis, in comparison with a control group. Subjective data obtained with the Dizziness Handicap Inventory questionnaire were higher than the control groups, while no significant difference between the different groups was found in video Head Impulse Test (vHIT) results in terms of gain asymmetry. However, it is worth noting that the increase in vHIT overt and covert saccades was in a direct relationship with the creatinine level and duration of the disease.
In light of these current suggestions, the aim of the present study was to investigate through the clinical use of vHIT the potential changes in vestibular function before and after haemodialysis, shedding light on a neglected topic among CKD patients.
Materials and methods
A prospective study was conducted at the Nephrology Unit and the Audiology and Phoniatrics Unit of the Magna Graecia University of Catanzaro over a 6-month period, from January to June 2024. Our cohort consisted of 18 adult patients diagnosed with stage 5 CKD and undergoing haemodialysis. The demographic breakdown of our study population was as follows: 11 males (61%) and 7 females (39%), with an age range of 45-75 years, and a mean age of 60 years. The clinical characteristics of the patients included a variety of comorbidities and previous treatments, which were carefully documented and taken into account in the analysis.
Inclusion criteria included: adult patients aged 18 years and over, diagnosed with stage 5 CKD and undergoing haemodialysis sessions in the Nephrology Unit during the study period, and a Visual Analogue Scale (VAS) score = 0 at the time of enrolment in the study.
Exclusion criteria encompassed: patients with current organic vestibular disorders, history of otological surgery, chronic ear disease, substance abuse, narcotic use, and those taking ototoxic and vestibulo-suppressant medications.
Vestibular function was assessed using the VAS 12 for the symptom of instability and vestibulo-ocular reflex (VOR) gain measured by the vHIT before (T0) and after (T1) the dialysis session. vHIT was performed using the ICS-impulse® device (GN Otometrics, 99 Taastrup, Denmark) for the lateral semicircular canals (LSC). vHIT is used to assess vestibular function by comparing head velocity to eye velocity when short, unpredictable head pulses are delivered. The vHIT measures the function of all 6 semicircular canals individually as head pulses are delivered in the horizontal, right anterior and left posterior (RALP) and left anterior and right posterior (LARP) plane.
Many studies suggest a reduced VOR gain in patients with vestibular dysfunction, and the lateral semicircular canal is the most clinically relevant because it is more commonly affected for anatomical reasons, i.e. the smaller number of afferent neurons condensed in a limited space 13-15. For this reason, we used the VOR gain of the lateral semicircular canal in our study.
Statistical analysis
The statistical software MedCalc version 19.1.7 (MedCalc Software bvba, Ostend, Belgium) was used to perform statistical analyses. The t-test and the Welch test were used to compare the numerical values at T0 and T1. Additional analyses were performed to ensure the robustness of the results. These included a paired sample t-test to compare the means of related groups and a Wilcoxon signed-rank test to assess differences between paired groups of non-normally distributed data. The Chi-square test was also used to examine the relationship between categorical variables. A p value of less than 0.05 was considered statistically significant.
Results
The results showed that at T1, 22% of patients (n = 4) had a VAS of 1 to 3, 56% (n = 10) had a VAS of 4 to 6, and 22% (n = 4) had a VAS of 7 to 10.
With vHIT, the mean VOR gain threshold decreased from T0 (0.99) to T1 (0.92) with a statistically significant difference (p = 0.034 and p = 0.037). These results are presented in Tables I, II, and III, Figure 1 and Cover figure to provide a clear visual representation of the results.
Discussion
Our study provides valuable insights into the impact of haemodialysis on vestibular function in patients with CKD, showing a significant reduction in vestibular function as seen by the decrease in VOR gain and increase in VAS instability scores. These results are consistent with previous reports that have highlighted an impairment in audiovestibular function in patients with CKD.
In particular, patients with CKD have a higher risk than the general population of developing audiological conditions such as sensorineural hearing loss and tinnitus. These data add to the increased incidence in chronic renal disease of ENT disorders such as recurrent epistaxis, opportunistic infections and oropharyngeal candidiasis, changes in taste and smell, neck fasciitis, gingival hyperplasia, and xerostomia 16.
The current scientific literature suggests the involvement of the anterior labyrinth and the dysfunction of the inner hair cells (as demonstrated by otoacoustic emissions) in CKD, also in relation to haemodialysis 17.
Although the first studies date back to the 1980s, the aetiology of inner ear dysfunction in CKD is still controversial and several factors may be implicated 18-21. Among them, uraemia-induced alterations in the central nervous system, called “uraemic neuropathy”, could cause dysfunction of the auditory nerve and central auditory pathways. It is worth noting that there are anatomical and physiological similarities between the nephron and the stria vascularis of the cochlea, as well as an immunological connection between the 2 organs. In this perspective, CKD may be associated with a disturbance in blood levels of Na+ and K+: both electrolytes are essential for the transmission of electrical signals within the auditory system and for the normal electromotility of the inner hair cells. Among the structural and functional similarities of the kidney and cochlea, the most relevant is, in fact, the active transport of electrolytes and fluids conducted in the glomerular basement membrane and in the cochlear vascular stria due to the presence of the ATP-dependent Na+/K+ pump and the carbonic anhydrase enzyme. The alteration of the electrolyte balance that occurs in patients suffering from CKD can therefore lead to changes in the stria vascularis and disturbance of the cochlear fluids (alteration of potassium recycling in the scala media and change in the ionic concentration in the perilymph and endolymph) with poor coupling of energy from the receptor to the hair cells.
However, scientific reports investigating vestibular function in CKD are still scarce, especially in relation to treatment 6. Existing evidence has shown that a glomerular filtration rate (GFR) < 60 mL/min is more associated with the possibility of developing vestibular alterations compared to the healthy population. Therefore, a negative correlation between GFR and vestibular function has been detected, with a notable impact on the risk of falls, a topic that significantly affects the CKD population.
Although the exact cause of vertigo symptoms in these patients remains unclear, potential aetiological factors are retention of toxic products resulting in vasculopathy, vestibulocochlear neuropathy, and vascular calcification in the labyrinth. It is also possible that changes in the sympathetic control of blood pressure may occur, which could influence the blood supply to the inner ear, leading to cochleo-vestibular dysfunction. Finally, medical therapy usually involves ototoxic drugs, in particular loop diuretics and aminoglycoside antibiotics, which cause electrolyte excretion and changes in endolymphatic and perilymphatic fluids composition.
Another interesting hypothesis has been also borrowed from neurology and translated to audiovestibular pathology. Dialysis disequilibrium syndrome, defined initially in 1962 22 is an acute neurological complication of hemodialysis characterised by signs and symptoms that can include fatigue, nausea, vomiting, blurred vision, tremors, disturbed consciousness, coma and also death due to cerebral oedema. The neurological symptoms are more common toward the end of a dialysis treatment that is characterised by rapid clearance of urea in which accentuates the plasma cerebrospinal fluid urea concentration gradient. The term disequilibrium is employed because the syndrome typically occurs when the blood biochemistry parameters are improving.
It is reasonable to hypothesise a similar mechanism underlying the possible presence of persistent apogeotropic direction-changing positional nystagmus when rapid removal of blood urea causes reduction of serum osmolality in patients on haemodialysis 9,23. The process is developed in 2 phases: when the decrease in osmotic pressure is greater than the decrease in hydrostatic pressure after haemodialysis, water moves from the inner ear capillaries to the perilymph, lowering the density of the perilymphatic fluid; subsequent water passage from the perilymph to the endolymph lowers the density of the endolymph below that of the cupula.
Objective data about vestibular dysfunction have been described in the work of Gabr et al. 24, in which the absence of cVEMPs and oVEMPs was demonstrated in 40% and 70% of patients with CKD respectively, regardless of whether they had undergone conservative or haemodialysis treatment. P13-N23 amplitude reduction was reported in both study groups compared to the control group.
The present preliminary study sheds light on subjective and objective vestibular data in relation to haemodialysis treatment. The distribution of VAS scores at T1 shows a significant variation in patients’ perception of instability, suggesting that haemodialysis may have a different effect on the stability of individual patients. This is in line with the study by Caplin et al. 25 who reported that approximately 60% of patients on haemodialysis experience dizziness, a symptom that can be attributed to various factors such as medication, blood volume depletion, hypotension, anaemia, malnutrition, dialysis imbalance syndrome and inappropriate dialysate sodium concentrations. It is reasonable to think that the modification of concentrations in the perilymph and endolymph causes poor coupling of energy from the otolithic membrane of the macula of the saccule to the hair cells.
Our findings are further supported by the work of Sazgar et al. 7 who demonstrated the effects of end stage renal disease on the auditory and vestibular systems, with the otolith organs playing a critical role in stabilising the body and gaze. This is consistent with another study by Varghese et al. 10 who compared otolith function in individuals with CKD to those without and showed abnormal vestibular evoked myogenic potential findings in the first population. These studies demonstrated considerably higher VEMPs impairment rates in patients with increased disease duration and higher creatinine levels after haemodialysis, although it is known that the creatinine level per se is not indicative of dialysis efficiency.
In this light, there is a high connection with our results, as they suggest as a future perspective to study the relationships between vestibular function and other indicators of disease progression and dialysis efficiency, such as the urea reduction ratio.
Limits of the study
Our study has some limitations; the small sample size of the cohort may limit the statistical power of our findings and the ability to detect significant differences between groups, therefore the results of this study can only be considered preliminary. The lack of a control group of patients with CKD not undergoing haemodialysis makes it difficult to isolate the specific effects of haemodialysis on vestibular function. Furthermore, no VEMPs were performed.
From an instrumental point of view, the only test performed was vHIT on the lateral plane. This test only partially evaluates the vestibular function since it provides information about the integrity of the vestibular-oculomotor reflex for high-frequency stimuli. The choice to use only this test, which is very fast and not very demanding for the subject examined, can be understandable if one considers the fatigue experienced by patients at the end of a haemodialysis session, the end of which it would undoubtedly be complex to evaluate patients using bithermal caloric testing, VEMPs or stabilometric tests.
In addition, our study did not evaluate the long-term effects of haemodialysis on vestibular function, which is an important consideration in the overall management of patients with CKD. Finally, the study did not investigate the underlying mechanisms by which haemodialysis affects vestibular function, such as the specific changes in electrolytes levels and their effect on labyrinthine fluids in the inner ear.
Future research should address these limitations by conducting larger, multicentre studies with control groups and by investigating the long-term effects of haemodialysis on vestibular function in patients with CKD.
Conclusions
Our study provides preliminary evidence that haemodialysis may have a detrimental effect on vestibular function in patients with CKD. The significant decrease in VOR gain and the reported instability of VAS scores post-dialysis session highlights the potential impact of haemodialysis on the vestibular system. These findings have important clinical implications, as vestibular dysfunction can lead to symptoms of dizziness, imbalance and vertigo, significantly affecting a patient’s ability to perform daily activities and reducing their overall quality of life. Early detection of vestibular dysfunction can facilitate timely intervention, such as vestibular rehabilitation therapy, to reduce the risk of falls and improve the patient’s quality of life. Future research should explore the underlying mechanisms by which haemodialysis affects vestibular function, investigate the long-term effects of haemodialysis on vestibular function, and assess the potential benefits of interventions such as vestibular rehabilitation therapy in this patient population. The development of strategies to minimise the impact of haemodialysis on vestibular function could have a profound impact on the management of patients with CKD, ultimately improving their overall prognosis and quality of life.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
PV, PDL, AS, FR, FMG, RA, MB, MZ, EA, GC, MA, GC: conceptualization, Methodology, data sources, formal analysis, manuscript editing, writing and review, supervision and validation.
Ethical consideration
This study was approved by the Institutional Ethics Committee of Calabria Region (protocol number 262/2024).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki. Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: October 31, 2024
Accepted: January 13, 2025
Figures and tables
Figure 1. Average VOR Gain at T0 and T1.
| Instability | N | % |
|---|---|---|
| Mild (VAS 1-3) | 4 of 18 | 22 |
| Moderate (VAS 4-6) | 10 of 18 | 56 |
| Severe (VAS 7-10) | 4 of 18 | 44 |
| Patient n° | VAS T0 | Vas T1 |
|---|---|---|
| 1 | 0 | 3 |
| 2 | 0 | 5 |
| 3 | 0 | 2 |
| 4 | 0 | 7 |
| 5 | 0 | 5 |
| 6 | 0 | 4 |
| 7 | 0 | 8 |
| 8 | 0 | 2 |
| 9 | 0 | 6 |
| 10 | 0 | 6 |
| 11 | 0 | 4 |
| 12 | 0 | 5 |
| 13 | 0 | 8 |
| 14 | 0 | 3 |
| 15 | 0 | 4 |
| 16 | 0 | 6 |
| 17 | 0 | 8 |
| 18 | 0 | 5 |
| Patient n° | VOR GAIN left T0 | VOR GAIN left T1 | VOR GAIN right T0 | VOR GAIN right T1 |
|---|---|---|---|---|
| 1 | 1 | 0.89 | 1.09 | 0.97 |
| 2 | 0.92 | 0.92 | 1 | 0.88 |
| 3 | 0.97 | 0.8 | 1.14 | 0.83 |
| 4 | 0.71 | 0.74 | 0.87 | 0.87 |
| 5 | 0.95 | 0.98 | 1.21 | 1.17 |
| 6 | 0.74 | 0.73 | 1.13 | 1.02 |
| 7 | 0.73 | 0.83 | 0.92 | 1 |
| 8 | 0.84 | 0.87 | 1.02 | 0.98 |
| 9 | 0.95 | 0.9 | 1.56 | 0.85 |
| 10 | 1.26 | 0.96 | 1.16 | 0.64 |
| 11 | 0.95 | 0.96 | 1.1 | 1.1 |
| 12 | 0.96 | 0.9 | 1.03 | 0.98 |
| 13 | 1.15 | 0.86 | 1.12 | 1 |
| 14 | 0.69 | 0.79 | 0.89 | 0.89 |
| 15 | 0.94 | 1.05 | 1.06 | 1.14 |
| 16 | 0.82 | 0.82 | 0.97 | 0.99 |
| 17 | 0.93 | 1.03 | 1.02 | 1.11 |
| 18 | 1.05 | 0.91 | 0.99 | 0.87 |
References
- Elliott D. Hemodialysis. Clin Tech Small Anim Pract. 2000;15:136-148. doi:https://doi.org/10.1053/svms.2000.18297
- Locatelli F, Canaud B. Dialysis adequacy today: a European perspective. Nephrol Dial Transplant. 2012;27:3043-3048. doi:https://doi.org/10.1093/ndt/gfs184
- Davenport A. Role of dialysis technology in the removal of uremic toxins. Hemodial Int. 2011;15:S49-S53. doi:https://doi.org/10.1111/j.1542-4758.2011.00602.x
- Maduell F, Rodríguez-Espinosa D, Jesus Broseta J. Latest trends in hemodiafiltration. J Clin Med. 2024;13. doi:https://doi.org/10.3390/jcm13041110
- Lee S. Potential role of psychosocial factors on health-related quality of life in hemodialysis patients. J Korean Med Sci. 2018;33. doi:https://doi.org/10.3346/jkms.2018.33.e121
- Jung D, Lee K, Do J. Chronic kidney disease as a risk factor for vestibular dysfunction. Postgrad Med. 2017;129:649-652. doi:https://doi.org/10.1080/00325481.2017.1338493
- Sazgar A, Ahmadi F, Akrami K. Vestibular evoked myogenic potentials of haemodialysed patients with end stage renal disease. Eur Arch Otorhinolaryngol. 2008;265:393-396. doi:https://doi.org/10.1007/s00405-007-0457-z
- Davenport A, Vervloet M. Hemodiafiltration. (Nubé M, Grooteman M, Blankestijn P, eds.). Springer; 2016. doi:https://doi.org/10.1007/978-3-319-23332-1_11
- Kim C, Shin J, Park J. Dialysis disequilibrium syndrome revisited: feeling “Disequilibrated” due to inner ear dyshomeostasis?. Med Hypotheses. 2019;129. doi:https://doi.org/10.1016/j.mehy.2019.109262
- Varghese S, Kumar K, Kalaiah M. Vestibular evoked myogenic potentials in chronic renal disease. Acta Otolaryngol. 2021;141:925-928. doi:https://doi.org/10.1080/00016489.2021.1983212
- Ozmen A, Ozer F, Torun D. Audiological and vestibular measurements in chronic renal failure patients receiving hemodialysis treatment. J Int Adv Otol. 2024;20:50-56. doi:https://doi.org/10.5152/iao.2024.231235
- Delgado D, Lambert B, Boutris N. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2. doi:https://doi.org/10.5435/JAAOSGlobal-D-17-00088
- Cherchi M, Yacovino D. Histology and neuroanatomy suggest a unified mechanism to explain the distribution of lesion patterns in acute vestibular neuropathy. Exp Brain Res. 2021;239:1395-1399. doi:https://doi.org/10.1007/s00221-021-06094-9
- Viola P, Marcelli V, Sculco D. Vestibular disorders after kidney transplantation: focus on the pathophysiological mechanisms underlying the vertical nystagmus associated with tacrolimus-related hypomagnesamia. Int J Environ Res Public Health. 2022;19. doi:https://doi.org/10.3390/ijerph19042260
- Alfarghal M, Algarni M, Sinha S. VOR gain of lateral semicircular canal using video head impulse test in acute unilateral vestibular hypofunction: a systematic review. Front Neurol. 2022;13. doi:https://doi.org/10.3389/fneur.2022.948462
- Krajewska Wojciechowska J, Krajewski W, Zatoński T. Otorhinolaryngological dysfunctions induced by chronic kidney disease in pre- and post-transplant stages. Eur Arch Otorhinolaryngol. 2020;277:1575-1591. doi:https://doi.org/10.1007/s00405-020-05925-9
- Kang S, Lim H, Yu H. Idiopathic sudden sensorineural hearing loss in dialysis patients. Ren Fail. 2018;40:170-174. doi:https://doi.org/10.1080/0886022X.2018.1450760
- Antonelli A, Bonfioli F, Garrubba V. Audiological findings in elderly patients with chronic renal failure. Acta Otolaryngol. 1990;476:54-68. doi:https://doi.org/10.3109/00016489109127256
- Bergstrom L, Thompson P, Sando I. Renal disease. Its pathology, treatment, and effects on the ear. Arch Otolaryngol. 1980;106:567-572. doi:https://doi.org/10.1001/archotol.1980.00790330047014
- Ikeda K, Kusakari J, Arakawa E. Cochlear potentials of guinea pigs with experimentally induced renal failure. Acta Otolaryngol. 1987;435:40-45. doi:https://doi.org/10.3109/00016488709107349
- Kim J, Lee S, Cha J. Chronic kidney disease is associated with increased risk of sudden sensorineural hearing loss and Ménière’s disease: a nationwide cohort study. Sci Rep. 2021;11. doi:https://doi.org/10.1038/s41598-021-99792-x
- Kennedy A, Linton A, Eaton J. Urea levels in cerebrospinal fluid after haemodialysis. Lancet. 1962;279:410-411. doi:https://doi.org/10.1016/S0140-6736(62)91365-X
- Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: a narrative review. Semin Dial. 2008;21:493-498. doi:https://doi.org/10.1111/j.1525139X.2008.00474.x
- Gabr T, Kotait M, Okda H. Audiovestibular functions in chronic kidney disease in relation to haemodialysis. J Laryngol Otol. 2019;133:592-599. doi:https://doi.org/10.1017/S0022215119001415
- Caplin B, Kumar S, Davenport A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant. 2011;26:2656-2663. doi:https://doi.org/10.1093/ndt/gfq763
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 617 times
- PDF downloaded - 209 times



