Thyroid
Vol. 45: Issue 3 - June 2025
Surgical approaches to the management of the intrathoracic goiter – A systematic review
Abstract
Objective. Intrathoracic goiters (ITGs) pose numerous challenges to head and neck surgeons due to the intricate relationships with major vessels and other mediastinal structures. Surgical excision remains the mainstay of treatment and we herein present an update on this topic.
Methods. A systematic review from 2017 to date was performed in the PubMed database and a total of 93 articles were identified and discussed, along with methodological issues and future directions in the research on ITGs.
Results. Transcervical excision is the commonest approach for treating ITGs, yet the potential need for a transthoracic approach must be always kept in mind. An acceptable rate of postoperative complications is expected if surgeries are carried out by experienced and dedicated surgical teams.
Conclusions. Surgical excision remains the principal treatment for ITGs and new less invasive techniques are being developed. Surgery for ITGs should be always carried out in specialist centres with experienced multidisciplinary teams.
Introduction
Multinodular goiter is defined as an enlarged thyroid gland with multiple nodules 1. It affects 4% of the US population and 10% of the British population and is estimated to affect 1.5 billion people globally 1. Iodine-deficiency contributes to the vast majority of cases of goiters worldwide, resulting in a presumed over-secretion of thyroid stimulating hormone (TSH). This results in a benign proliferation of hyperplastic follicles and adenomatoid nodules, some with cystic degeneration 2. Intrathoracic goiter (ITG), also referred to as substernal or retrosternal, or cervico-mediastinal goiter, was first described by Haller in 1794. It represents a challenge for surgeons, as well as for anaesthesiologists 3,4.
Most patients present with shortness of breath on exertion although up to a third of patients with ITG may be asymptomatic and the goiter is an incidental finding. Patients may experience tracheal deviation, external compression, and even risk of tracheomalacia and cardiovascular collapse (Cover figure). ITGs should therefore be managed only in referral centres where such expected difficulties can be safely managed. In this regard, an accurate preoperative work-up is mandatory in order to identify adverse factors, minimise complications and potentially reduce the need for an extra-cervical approach (ECA) 2.
The most recent review available on ITG dates to 2018, yet recent advances in medical technology have brought innovations, especially with new mini-invasive techniques such as image-guided ablation or robotic-assisted transthoracic surgery 5.
The present review aims to systematically appraise the most recently published literature on the surgical treatment of ITGs, with particular attention to the multidisciplinary management of these patients.
Materials and methods
The PRISMA statement was followed in the preparation of the present paper, and a modified PRISMA flowchart is given in Figure 1 6. No institutional review board approval was necessary for the present work. The PICOS methodology was used with the main question being “What are the current surgical treatment options for managing intrathoracic goiter?”. In detail, P(atients): patients diagnosed with ITGs; I(ntervention): surgical treatments; C(omparator): none; O(utcomes): resolution of symptoms and rates of post-treatment complications; S(tudy design): reports, retrospective or prospective cohort studies, and randomised clinical trials.
The PubMed database was used in order to perform the review of the literature from January 1 2017 to January 1 2024. The following search string was used: “intrathoracic” OR “retrosternal” OR “cervico-mediastinal” OR “substernal” AND “goiter” OR “goiter”. All the pertinent articles were included after careful reading of titles and abstracts. Full texts of the included articles were then retrieved by the first co-author (LGL) and quantitative and qualitative data were summarised accordingly. Papers reporting surgical outcomes of ITGs mixed with other thyroid disorders (Reason 1); articles reporting non-surgical outcomes of ITGs (Reason 2), or written in languages other than English, French, or Italian (Reason 3) were excluded.
The search strategy retrieved a total of 239 articles and, after applying the selection criteria, a total of 91 articles were analysed. Additionally, a further 2 articles were retrieved and included in the discussion after checking through the reference lists of the relevant studies. Quantitative and qualitative data regarding surgical outcomes were summarised and systematically reported in tables.
Results and discussion
Definition and classification of ITG
Etymologically, ITG must at least partially be located in the mediastinum. However, a myriad of factors affects this seemingly simple definition: clinical versus radiological evaluation, head extension at the time of examination, the proportion of thyroid mass that is required to extend into the thoracic cavity, etc. As of today, there is still no commonly accepted definition of ITG, which appears to suffer from an “identity crisis” 4. A recent historical overview describes over 10 definitions for ITGs 7. Notably, Rios et al. analysed the various criteria to define ITGs and found none to be clinically meaningful 8. The most recent definition comes from the 2020 American Association of Endocrine Surgeons (AAES) guidelines, where “if a mediastinal extension is present, meaning that the gland extends caudally past the sternal notch on physical examination, computer tomography (CT) imaging, or at the time of surgery” it can be considered as a true ITG. Since an authoritative international consensus has not been reached, the most recent literature is still heavily affected by these heterogeneous definitions (see Table I), and this represents an enormous obstacle for comparing surgical outcomes.
ITGs can be defined according to their extension and relationships with anatomical structures of the mediastinum: the most recent classification divides it into a Type I ITG (extending anteriorly to the trachea and the recurrent laryngeal nerve, RLN), a Type II ITG (where at least a part of the mass extends posteriorly to the mediastinal vessels, the trachea, and the RLN), and a Type III (purely mediastinal) ITG9. Unfortunately, a formal correlation of these classifications with surgical outcomes has not been yet performed. Over 99% of ITGs are secondary (Type I and II), i.e. they represent an inferior extension of the normal thyroid gland, and are vascularised by branches of thyroid arteries. Instead, primary (Type III) ITGs are clearly separated from the orthotopic cervical thyroid, they receive direct intrathoracic arteries, and are usually incidental findings 10. In these cases, a missed gland can be diagnosed only when postoperative TSH levels remain unchanged or a (functional) imaging of the chest is performed 11-14. Type III ITG is explained by an erroneous migration of the two median and lateral anlagen from the pharyngeal pouch and the ultimobranchial body 15. Migration of the median anlage may also lead to a presternal goiter, and in 2019 the seventh case worldwide was reported 16. Sometimes, after subtotal thyroidectomy, patients may present with a residual central mediastinal mass shifting the trachea laterally (“pseudo-primary ITG”) 17-19. This may also occur decades after the initial surgery and it may mimic other mediastinal or pulmonary pathologies, such as paraganglioma or adenocarcinoma of the lung 20,21.
Diagnostic work-up of ITGs
The diagnostic workup of a goiter should include standard thyroid function tests, a clinical examination of the neck, and airway assessment using transnasal fibreoptic laryngoscopy followed by neck ultrasound. Regarding physical examination, Pattashanee and colleagues have shown that clinical examination in the modified Rose position has an excellent sensitivity (98%) and a variable specificity (46.7-91.1%) to identify substernal extension 22. They demonstrated that in the absence of Pemberton’s sign, the probability of getting a false positive finding of an intrathoracic extension was only 4%, being relevant in the context of low-income countries where the availability of CT scan is scarce 22. Ziai et al. found that measuring thyroid volume by ultrasound may satisfactorily estimate both intrathoracic extension and tracheal compression: a cut-off value of ≥ 37.5 cm3 revealed an 83% sensitivity and 79% specificity for substernal extension (area under the curve [AUC] = 0.84), while with a threshold of ≥ 37.8 cm3 an 89% sensitivity and 87% specificity for any degree of tracheal narrowing were registered (AUC = 0.90) 23.
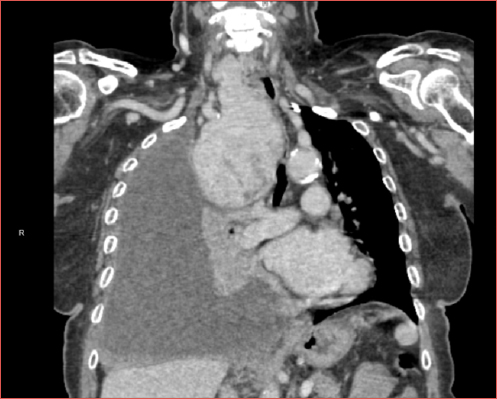
CT scan with iodine contrast remains the gold standard imaging investigation for the assessment and characterisation of ITG as it evaluates the dimensions, morphology, and relationships to the adjacent mediastinal anatomical structures (Fig. 2). The use of intravenous iodine contrast agents could theoretically precipitate acute thyrotoxicosis (Jod-Basedow phenomenon), although we could not find any recent reports documenting this 7,24. MRI and other cross sectional imaging techniques have not been shown to be of significant aid in the diagnostic work-up of ITGs. The same is true for nuclear medicine techniques, except from the aforementioned Type III ITG. Finally, the analysis of flow-volume loops during pulmonary function tests remains of very little significance and it is not indicated except for research purposes.
Work-up should be focused on the prediction of the feasibility of the transcervical approach. Some authors have proposed a simple yet not validated rule-of-thumb: if the ITG remains cranial to the aortic arch in the sagittal plane of CT performed while the patients maintain a full neck extension, then sternotomy is usually not necessary 25. Classical predictors of the need for sternotomy are based on the craniocaudal length of the retrosternal extension, the ratio between the retrosternal portion and the diameter of the upper thoracic inlet (so-called “iceberg” or “cone-shaped ITG”), and the anterior or posterior relationships with major vessels 4,26. Extension to or beyond aortic arch, shape and re-do or revision surgery are the most significant predictors for ECA 27. More recently a novel strategy based on volumetric analysis of the intrathoracic component has been reported: in a retrospective evaluation of 47 supine CT scans of ITG cases, a retrosternal thyroid volume of ≥ 162 cm3 had a negative predictive value for ECA of 100% 28. Volume was manually outlined on axial sections by two radiologists, but interrater agreement and kappa coefficients were not calculated, thus leaving doubts on the applicability of this technique.
Anaesthelogic considerations
ITG can be associated with significant laryngotracheal compression and deviation, resulting in challenging orotracheal intubation. Nonetheless, the majority of patients with ITG are amenable to endotracheal intubation, as the tube splints the trachea open at the area of maximal “soft” compression. Airway management, however, may be challenging 5,29,30. Many patients have variable symptoms related to head position: when the neck is fully extended, the goiter is pulled up towards the thoracic inlet, and the patient may complain of respiratory distress. In such cases, awake fibreoptic intubation is compatible with neck flexion during intubation 31,32.
Maintenance of anaesthesia implies no special requirements. At the end of surgery, tracheal compression resulting from long standing ITGs may cause a degree of tracheomalacia, although extubation is almost always possible. In the highly unlikely event that the patient suffers airway obstruction on extubation due to tracheomalacia, reintubation should be straightforward, and an elective tracheostomy can be performed at a later stage if necessary.
CT should provide a clear indication of laryngeal displacement, tracheal deviation, and extrinsic compression. This should alert the anaesthetic teams to consider endoscopic-assisted awake intubation. In very rare occasions some authors have reported the need for emergency tracheostomy 29, a paediatric flexible bronchoscope 33, or even a rigid bronchoscope to bypass the tracheal obstruction 30. In the most extreme case, the successful application of extracorporeal membrane oxygenation (ECMO) before general anaesthesia has been described 34. Actual evidence dispels the myth that ITGs constitute on their own a difficult airway case, and this is true in the absence of classical risk factors for difficult/failed intubation (mouth opening less than 4 cm, a thyromental distance less than 6 cm, Mallampati Class III or higher, etc.) 26. In a 2020 paper, 22 cases of “giant” Type I ITG (defined as trespassing the aortic arch on CT scan), with a mean maximum diameter of 114.2 ± 19.5 mm were all successfully intubated with standard laryngoscopy or awake tracheal intubation (for 5 patients, 22%) using flexible bronchoscopy 35. In a report from India, conventional unassisted intubation was achieved in 100% of patients with tracheal compression, including cases where “critical” narrowing (less than 5 mm) was documented on CT 31. A larger multi-institutional series from the US confirmed that direct laryngoscopy or videolaryngoscopy techniques were sufficient in 162 ITGs (90.5%), while transnasal or transoral fiberoptic intubation was used for the remaining 17 patients 36. The authors did not give a clear-cut definition of ITG, yet they performed a multivariate analysis where the body mass index was the only factor to positively correlate with the number of attempted intubations 36. This was also confirmed in a series from Olusomi et al., which showed that intrathoracic extension did not predict the risk for failed or difficult intubation, whereas neck circumference (indirectly linked to the fat mass) did 32.
Surgical indications
Classical indications for surgically removing non-toxic multinodular goiters date back to the beginning of the late 19th century: relief from compression symptoms, prevention of or suspicion of an associated malignancy, or for cosmetic purposes 37. Unfortunately, these criteria are somehow vague and relative. In 2018 a “choosing wisely” initiative from Germany proposed more reproducible surgical indications: compression symptoms (dyspnoea, stridor, dysphagia) must be directly attributable to goiter, while a reasonable suspicion of malignancy must be documented (i.e., TI-RADS category 4c/5, Thy 3 or higher class at FNAC in the presence of risk factors for thyroid cancer, basal calcitonin serum level increase > 26 pmol/L in women and 60 pmol/L in men, cN+ status at ultrasound). In the absence of the above, surgery may also be indicated for “prevention of complications’’ that may derive from a progressive ITG (defined as tracheal compression > 35% or superior vena cava syndrome)” 38. The progression must be documented, but the very natural history of untreated ITG is still poorly understood 4.
Classical surgical approaches to ITG
An exhaustive explanation to the patient is key to surgical success 5. The classical Kocher incision may be extended vertically in a T-shape fashion, and this occurred in 17 of 265 (6.4%) cases in a series published in 2024 39. Transection of the pre-laryngeal musculature is also often necessary for delivering large goiters, and a study in 2022 for the first time evaluated its consequences on vocal and swallowing function: by using patient-reported impairment scores, a prospective head-to-head study of 34 patients revealed no significant differences when strap muscles are transected or not 40.
In 2021, the International Neural Monitoring Study Group (INMSG) published a key document on surgical anatomy in the era of intraoperative neuromonitoring (IONM). The authors meticulously evaluated the course and anatomical relationships of 1000 RLNs: strikingly, among other findings, it emerged that in 50% of cases of ITGs (versus 30% in standard thyroidectomies), the RLN was fixed, splayed, or entrapped at the level of the capsule of the thyroid, thus rendering at high risk for loss of signal 41. IONM is thus highly advisable for ITG surgery and, in the case of “giant” ITG, many authors suggest the upfront use of a transcervical “medial” approach, which recommends the division of the isthmus and dissection of Berry’s ligament in a layered fashion along the trachea and towards the RLN. In a series from the US, successful identification of the RLN was reported to be 84% with this technique and postoperative vocal cord dysfunction (assessed by flexible laryngoscopy) was null 42. A very high rate (17%) of postoperative hematoma was reported, but surprisingly the authors declared that surgical revision was necessary for only one patient 42. Another larger case series from Greece reported no postoperative bleeding, and a permanent RLN damage rate comparable with the traditional “lateral” approaches 43.
Controversy exists regarding the extent of the resection: total thyroidectomy remains the preferred approach for ITGs that are affecting both lobes 24. It must be always taken in account that ITGs can harbour one or more foci of malignancy in up to 40% of cases, even though on average this seems to be around 10% (Tab. I). When the intrathoracic part is well lateralised, or there are anatomical constraints or technical issues during the dissection, a subtotal resection or a simple hemithyroidectomy may help relieve the compression symptoms while minimising postoperative complications. New technologies are being exploited to facilitate resection of ITGs: Dagan et al. proposed a microdebrider-assisted intracapsular reduction of the goiter in a small series of 26 patients 52. By a standard sinus suction debrider the authors did morcellate the intracapsular part of the ITG; they reported that it has no effect on the risk of major bleeding, and it may avoid the need for sternotomy in selected patients 52.
The most recent works on transoral endoscopic thyroidectomy vestibular approach (TOETVA) and transoral robotic thyroidectomy (TORT) consider ITG as an exclusion criterion because of intrinsic limitations in terms of surgical exposure 68. Finally, the well-known consequences of permanent postoperative hypothyroidism must be considered when this operation is undertaken in underdeveloped countries 69. In a series of 160 patients from Kenya, an ITG was present in 7 (3.8%), although total thyroidectomy was performed in only 5 cases. In an algorithm for avoiding the need for lifelong L-thyroxin therapy, the authors did not include the substernal extension of the goiter as a determining factor 69. The issue of the extension of the thyroidectomy should be addressed in future studies because ITGs are frequently encountered in developing and low-income countries where goiters are endemic 70.
Current indications for extra-cervical approaches (ECA)
Several goiter-related and patient-related factors must be considered in the surgical plan for every ITG. Patients are deemed at high risk for ECA when: 1. ITGs extend below the aortic arch; 2. they present multiple or separate mediastinal compartments; or 3. they show an “iceberg” or conical shape (i.e., extending to both sides of the thorax or with a diameter that is larger than the upper thoracic inlet) 27. ECA are also indicated in revision surgery as fibrosis in the thoracic inlet may impair successful dissection of the intrathoracic component of the goiter 5,24,25,71. Some authors believe that in the presence of possible malignancy, sternotomy may be favoured, but there is no consensus on this point64. Furthermore, this risk is low on average (Tab. I) and most ITGs are not actually suitable for biopsy because nodules are difficult to reach, and with the risk of puncturing mediastinal vessels or pleura. Additionally, the vast majority of the malignancies are incidental foci of differentiated carcinoma: for example in a recent paper, the mean dimensions of the tumours were 5.6 ± 2 mm (i.e., pT1) 44; others instead did not report the TNM or nodal status 67. In extreme cases, benign ITG may be strictly adherent to the pericardium 72, or it can even invade it (an exceedingly rare “intrapericardial ITG”) and sternotomy as well the availability of a cardiopulmonary bypass is essential 73.
Patient-related features that may favour ECA include a history of previous thyroid or thoracic surgery as indicated, previous irradiation in the neck or chest areas, preoperative dysfunction of the vocal cords, coagulopathies or platelet disorders (e.g., Bernard-Soulier syndrome) 4,24,74. In a recent meta-analysis on over 3000 patients, approximately 6% of ITG needed to undergo an ECA 75.
A recent study that has corroborated the predictive factors for ECA was published in 2019: in a cohort of 237 ITGs where 29 (12.2%) required sternotomy, on multivariate logistic regression analysis, extension below the aortic arch (OR= 10.84), an iceberg shape (OR= 59.30), and previous neck surgery (OR= 4.83) were all significantly associated with an ECA 27. Casella et al. also found the presence of an inflammatory component in the excised ITG to be a predictor for sternotomy (only on univariate analysis and in a small series) 76. In another series of 109 ITGs, only the part extending beyond the sternal notch into the mediastinum predicted sternotomy (univariate only, OR= 3.43, CI= 1.65-6.41), with a sensitivity of 94% and specificity of 86.5% when it is more than 5 cm 77. Unaccountable factors such as the surgeon’s expertise do however exist. There are reports where ITGs extending clearly below the aortic arch or in the posterior mediastinum were completely excised through a purely transcervical approach 54.
Complete sternotomy is the gold standard access for ITGs, but it can be very painful and with the risk of serious wound complications. To overcome this, less extensive approaches have been devised, namely partial or split sternotomy 78,79, anterolateral or posterolateral thoracotomy, the hemi-clamshell approach (partial median sternotomy plus an anterolateral thoracotomy) 80, and they are all technically described in the work by Uludag et al. 26. Endoscopic-assisted approaches are another option but in the last years, video-assisted mediastinoscopy has lost its interest in favour of thoracoscopic approaches 5,81. For example, the subxiphoid thoracoscopic approach is a novel alternative proposed by a Chinese group for median ITGs 48. Robotic-assisted procedures are being also actively explored, but they are always combined with a transcervical incision 82-84.
In the 2016 American Thyroid Association (ATA) Statement on Remote-Access Thyroid Surgery (RATS), the substernal extension was considered a contraindication, but the latest papers seem to overcome this limit. A RATS by an axillo-thoracic endoscopic approach has been applied on very selected patients with ITG 85. The excellent view of the surgical field provided by high-definition cameras is obviously very helpful in the identification of critical neurovascular structures (vagal and phrenic nerves, etc.) but, at present, all these approaches remain preliminary and a formal comparison with standard techniques is lacking. In very rare cases, a straightforward indication for ECA is given by a coexisting thoracic condition: for instance, a minimally invasive transcervical and robotic transthoracic approach has been described for ITG and concurrent thymoma 86, or ITG excision and aortic valve replacement were performed through a mini-J sternotomy 87.
Finally, in case of ITGs encroaching the epiaortic vessels (or whenever there is preoperative suspicion of malignancy or other histology than ITGs), the older transclavicular approaches are being replaced by less invasive techniques. For instance, the transmanubrial osteomuscular-sparing approach (TOSA) does not affect the sternoclavicular joint, clavicle, or the pectoralis major muscle and it offers a superb view of the neurovascular structures of the superior and anterior mediastinum with minimal morbidity, as was summarised in a recent review 88. Intuitively, mastering all of these techniques lets an experienced multidisciplinary team manage even the most challenging cases. Future studies validating the classification of ITGs are also anticipated in order to corroborate the risk factors for combined surgical approaches.
Complications associated with surgery for ITG
The presence of ITG is historically known to carry a higher risk of finding a malignancy and of the develop postoperative complications (haematoma, transient/permanent hypoparathyroidism, RLN dysfunction, etc.) compared to cervical goiters 89. Notably, the expertise of the surgeon remains the best protective factor for reducing this risk. In a recent analysis of the United Kingdom Registry of Endocrine and Thyroid Surgery, a high (> 100 cases/year per surgeon) annual operative volume was a key factor in minimising adverse outcomes from thyroid surgery 74.
A summary of the reported postoperative complications is given in Table II and a pooled rate of 3.7% was estimated. Van Slycke and colleagues reported that the higher risk of postoperative complication is influenced by the surgical approach chosen: out of 95 thyroidectomies performed for ITGs, 80 patients (84%) were operated by cervicotomy and 15 (16%) by cervicosternotomy. The latter group had a higher risk of temporary recurrent laryngeal nerve palsy (21%) compared to cervicotomy (4%) and standard thyroidectomy (3%); also, the risk of temporary hypocalcaemia after cervicosternotomy was higher than with transcervical approaches 64. In addition, another study highlights how postoperative complications associated with ITG surgical removal are low in the hands of experienced, high-volume thyroid surgeons, regardless of transcervical or transthoracic approach 62. Instead, the presence of ITG was significant for being an independent predictor of any complication (OR= 2.1), when controlling for age, BMI, gender, and race 62.
INJURY TO RLN
In a cohort of 1,500 RLNs at risk, substernal extension and a thyroid volume > 100 mL were the only significant factors for postoperative transient or permanent VC paralysis 60. In the IONM era, a study extracting 42,341 operations from the UK Thyroid database found that ITG was an independent factor (OR= 1.36, 95% CI 1.05-1.77) that predicted the risk of RLN injury, along with revision surgery and patient age 74. A recent anatomical study has revealed that iatrogenic lesions of the RNL after ITG resections are more frequent on the right side in comparison to the left because of the known anatomical and embryological differences in the development of the RLN on either side of the neck 90.
POSTOPERATIVE HAEMORRHAGE
Tausanovic et al. found that male gender, use of preoperative anticoagulant therapy or postoperative subcutaneous heparin, and presence of ITG (38 cases) were all significant predictors of the development of a postoperative haematoma, in a group of 6,900 thyroidectomies 91. ITG was not a significant risk factor in another series of 5,900 operations, including a total of 148 ITGs (2.5%) 92. Both series, unfortunately, did not provide a definition of ITG nor how the extension was determined. On the contrary, another analysis of the aforementioned UK registry (n= 53,838 entries) revealed in multivariable analysis that male gender, increasing age, re-do surgery, ITG (whenever having an “extension to the thoracic inlet or below”), and total thyroidectomy were all correlated with an increased risk of reoperation for bleeding; surgeon monthly thyroidectomy rate correlated with a decreased risk 93.
HYPOPARATHYROIDISM AND HYPOCALCAEMIA
The presence of ITG is linked with a major risk of developing clinical symptoms of hypocalcaemia (OR= 10.26) 94. A recent study published by Chen et al. provides evidence that there are predictive factors for this adverse event. This meta-analysis includes 23 studies and identified 12 significant risk factors for postoperative hypocalcaemia 95. In particular, hypoparathyroidism (OR= 5.58), total thyroidectomy (OR= 3.59), hypomagnesaemia (OR= 2.85), and preoperative vitamin D deficiency (OR= 2.32) were those most associated with hypocalcaemia 95. In another observational study, ITG and its extension beyond the carina showed a significantly higher risk for transient (less than one year) hypocalcaemia (relative risk = 1.76) after total thyroidectomy 61.
TRACHEOMALACIA
Tracheomalacia remains one of the most challenging complications in large ITG surgery, even though its incidence is reported to be very low 51. In a series of 40 patients subjected to thyroidectomy with sternotomy, tracheomalacia was reported in 3 cases, in absence of any patient- or thyroid-related factor significantly associated with its development: one patient required tracheal resection with anastomosis, and 2 patients required tracheostomy 51. In another paper, 17 cases of tracheomalacia out of 106 cases were reported, probably caused by long-standing tracheal compression from ITG: this study suggested that keeping patients under prolonged intubation was sufficient to resolve airways collapse without tracheal reconstruction in all cases 53. Zuo et al. reported how tracheomalacia could be also successfully managed intraoperatively by suspending the trachea to the overlying skin with Prolene stitches, despite the fact that they did not mention either the exact number of patients affected or the long-term outcomes 96.
LENGTH OF STAY
Most patients undergoing surgery for ITG will stay several days in hospital to recover. Simo et al. found that the length of stay was significantly prolonged by the need of an ECA (3 days vs 6 days) 5. However, ITG was not considered a contraindication for outpatient surgery: in a recent national survey conducted by the American Association of Endocrine Surgeons, the diagnosis of ITG had no or minimal effect on same-day discharge 97.
POSTOPERATIVE INFECTION
Infection rates are low in thyroid/parathyroid surgical procedures as they are considered clean surgeries. Recently, the use of ECA seems to be associated with a higher risk of infection (1.4% vs 0.2% in transcervical resection, p = 0.006), in a recent international series 47.
The main issue in the analysis of the complications of surgery for ITGs remains their non-standard definitions. For instance, postoperative vocal cord dysfunction may be diagnosed by using a mirror 65, flexible laryngoscopy 42, or a mixture of both 60. It is well known that RLN dysfunction rates vary according to the method of examining the larynx and range from 26% to 2.3% 98. Another example is the timing of what constitutes a “permanent” hypocalcaemia change (after 6 42 versus after 12 months 67). To overcome these limitations, the use of the Clavien-Dindo system may be a solution: proposed in 1992 and revised in 2004, it appears to be reliable as a compelling tool for quality assessment in surgery worldwide. This classification system was reported as simple, reproducible, logical, useful, and comprehensive 99, and it is beginning to be implemented in thyroid surgery 100.
Conclusions
ITG is a relatively frequent condition for head and neck and endocrine surgeons. Its management requires a systematic and multidisciplinary approach including US and multiplanar CT scan with contrast. Patients undergoing surgery for ITGs will require dedicated anaesthesiological, endocrine and surgical teams. Approximately 10% of patients will require ECA, especially when ITGs extend below the aortic arch, have an iceberg shape, or in case of revision surgery. The rate of complications appears to be higher than in standard neck surgery, but future studies and international consensus on these procedures are advisable to standardise terminology, approaches, and reporting of outcomes.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
CM, LGL: conceptualisation, writing original draft; NC, RS, AMB-B, SR, MGR: revising original draft.
Ethical consideration
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki. No patients or participants were involved in the present study.
History
Received: November 3, 2024
Accepted: December 21, 2024
Figures and tables
Figure 1. A modified PRISMA flowchart for selection and inclusion of the relevant studies in the present review.
Figure 2. High resolution CT sequences highlighting a bulky goiter extending in the mediastinum. A-B) axial scans; C) sagittal scan; D) coronal scan. A mild tracheal compression can be noticed (*).
| Author | Number of patients, gender distribution, and mean age (years) | Number and percentage of ITG | Malignancy rate at final histopathology | Working definition of ITG | Preoperative workup | Surgical approach chosen | Type of surgery | Mean operating time (minutes) | Mean length of hospital stay (days) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sahbaz et al. 44 | 1270 (310 M, 960 F); 47.5 | 390 (30.7%) | 76 (19%) | “goiters that reach below the thoracic inlet or a thyroid mass that has 50% or more of its volume located below the thoracic inlet” | NA | 30 TransTx 1240 TransCx | 1270 (100%) TT | NA | NA | |
| Tostado et al. 45 | 60 (7 M, 53 F); 58 | 60 (100%) | 9 (15%) | “the presence of thyroid tissue beyond the thoracic inlet” | 100% US 100% CT 68.3% FNA | 2 (3.3%) TransTx 58 (96.7%) TransCx | 47 (78.4%) TT 11 (18.3%) STT 2 (3.3%) HT | NA | NA | |
| Welman et al. 46 | 35 (8 M, 27 F); 67.4 | 35 (100%) | 0 (0%) | “thyroid mass that extends 3 or more cm below the suprasternal notch when the neck is hyperextended” | 100% CT | 8 (22.8%) TransTx 27 (77.2%) TransCX | 100% TT | 131.7 | 5.5 | |
| Cappellacci et al. 47 | 4467 (1068 M, 3399 F); 53.55 | 276 (6.2%) | 1656 (37.1%) | “thyroid in which any part of the gland extended below the thoracic inlet with the patient in the surgical position with the neck in hyperextension” | NA | NA | 100% TT | 117 | 2.2 | |
| Wang et al. 48 | 10 (0 M, 10 F); 49.4 | 10 (100%) | NA | “the lower margin of the mass lower than the thoracic entrance” | 100% US 100% CT | 10 (100%) TransTx | 100% TT | NA | 4.5 | |
| Tsur et al. 39 | 265 (69 M, 196 F); 56.1 | 265 (100%) | 82 (31.3%) | “if more than 50% of the gland lay inferior to the thoracic inlet” | 100% US 100% CT | 3 (1.2%) TransTx 262 (98.8%) TransCx | NA | 117 | 4.9 | |
| Nakaya et al. 49 | 44 (11 M, 33 F); 60 | 44 (100%) | 5 (11.4%) | “thyroid gland with more than 50% of its mass located below the thoracic inlet” | 100% CT and/or MRI | 44 (100%) TransCx | 9 (20.5%) TT 35 (79.5%) HT | NA | NA | |
| Ghabisha et al. 50 | 28 (11 M, 17 F); 49.4 | 28 (100%) | 7 (25%) | “when the preoperative CT scan report indicated any extension of the goiter into the thorax through the thoracic inlet” | 100% CT | 28 (100%) TransCx | 28 (100%) TT | 121.9 | 5.3 | |
| Sulaiman et al. 51 | 40 (13M, 27F); 48.7 | 40 (100%) | 8 (2.5%) | NA | NA | 40 (100%) TranTx | 40 (100%) TT | NA | NA | |
| Dagan et al. 52 | 26 (7 M, 19 F); 65 | 26 (100%) | 1 (3.8%) | NA | 100% CT | 26 (100%) TransCx | 10 (38.5%) TT, 16 (61.5%) HT | NA | 3.2 | |
| Ren et al. 53 | 106 NA | 106 (100%) | NA | NA | 100% CT | 106 (100%) TransTx | NA | NA | NA | |
| Oukessou et al. 54 | 116 (24 M, 92 F); 47.6 | 116 (100%) | 10 (8.6%) | “a goiter extending below the plane of superior thoracic aperture on CT scan” | 100% CT | 116 (100%) TransCx | 105 (90.52%) TT 11 (9.48%) HT | NA | 3 | |
| Abdelrahman et al. 55 | 30, (18 M; 12 F); median 50 | 30 (100%) | 3 (10%) | “any goiter reported by the preoperative or intraoperative report to extend to the thorax through the thoracic inlet” | US 29 (96.7%); radioactive iodine study 9 (30%); CT 7 (23.3%) | 29 (96.7%) TransCx 1 (3.3%) TransTx | 17 (56.7%) TT 11 (36.7%) HT 2 (6.6%) STT | NA | 7.6 | |
| Aghajanzadeh et al. 56 | 70 (20 M; 50 F), NA | 70 (100%) | 28 (40%) | NA | 12 (17.1%) US; 58 (82.9%) CT | 67 (95.7%) TransCx 3 (4.3%) TransTx | 52 (74.3%) TT NA | NA | NA | |
| Battistella et al. 57 | 264 (53 M; 211 F); 54 | 264 (100%) | 10 (3.8%) | “thyroid tissue located at least 4 cm below the thoracic inlet” | 100% US CXR, 67% CT/MRI | 256 (97%) TransCx 8 (3%) TransTx | 135 (51%) TT 31 (11%) HT 98 (38%) STT | NA | 3 | |
| Chen et al. 58 | 227 (56 M; 171 F); 58 | 108 (47.6%) | 23 (10.1%) | “a goiter extending below the plane of the thoracic inlet on CT scan in the supine position” | 100% US + CT | 216 (95.2%) TransCx 11 (4.8%) TransTx | 136 (59.9%) TT 62 (27.3%) HT, 29 (12.8%) STT | NA | 8.7 | |
| Ching et al. 42 | 46 (12 M; 34 F); 60.9 | 46 (100%) | 3 (7%) | “at least 1 cm of extension inferior to the sternal notch” | 100% US + CT/MRI | 46 (100%) TransCx | 27 (58.7%) TT 19 (41.3%) HT | NA | NA | |
| Damiano et al. 59 | 58 (20 M; 38 F); 61 | 58 (100%) | 18 (31%) | NA | NA | 56 (96.5%) TransCx 2 (3.5%) TransTx | 58 (100%) TT | NA | 3.5 | |
| Doulaptsi et al. 43 | 212 (75 M; 137 F); 50 | 212 (100%) | 34 (16%) | “if any part of the gland extended through the thoracic inlet on imaging studies” | 100% CT and US | 211 (99.5%) TransCx 1 (0.5%) TransTx | 212 (100%) TT | NA | NA | |
| Głód et al. 60 | 830 (139 M; 691 F), 54.1 ± 14.2 | 171 (21%) | 76 (9.2%) | NA | NA | NA | 495 (59.6%) TT 160 (19.3%) HT 175 (21.1%) STT | 58.3 | NA | |
| Li et al. 61 | 142 (44 M; 98 F); 58 | 142 (100%) | 18 (12.6%) | “a goiter which extends at least 3 cm from the sternal manubrium when the patient is in an operative position and with a hyperextended neck” | NA | 140 (98.6%) TransCx 2 (1.4%) TransTx | 142 (100%) TT | 118 | 3.8 | |
| Linhares et al. 62 | 101 (26 M; 75 F); 56 | 101 (100%) | 19 (19%) | “if the gland extends caudally past the sternal notch on physical examination, CT imaging, or at the time of surgery” | 84% CT, 100% US | 86 (85.1%) TransCx 15 (14.9%) TransTx | 94 (93%) TT 7 (7%) HT | NA | NA | |
| Tabchouri et al. 63 | 70 (26 M, 44 F); 67 | 70 (100%) | 18 (25.8%) | “when more than 50% of the thyroid gland lies inferior to the sternal notch” | 100% US + CT | 54 (77.1%) TransCx 16 (22.9%) TransTx | 64 (91%) TT 6 (9%) HT | NA | 4.5 | |
| Van Slycke et al. 64 | 95 (40 M, 55 F); NA | 95 (100%) | 4 (5.8%) | “more than 50% of the thyroid is located below the thoracic inlet” | 100% US + CT/MRI | 80 (84.2%) TransCx 15 (15.8%) TransTx | 78 (82%) TT, 17 (18%) HT | 82 | 1.9 | |
| Vaiman et al. 65 | 70 (29 M, 41 F); 39 | 70 (100%) | 4 (5.8%) | “when the lower pole of the goiter was not palpable even after full head extension backward” | 100% US + CT/MRI | 69 (98.6%) TransCx 1 (1.4%) TransTx | 49 (70%) TT 21 (30%) HT | NA | NA | |
| Wang et al. 66 | 115 (21 M; 94 F); 52.9 | 115 (100%) | 6 (5.2%) | “extending below the thoracic entrance into the mediastinum” | 115 (100%) US 98 (85.22%) CT | 112 (97.4%) TransCx 3 (2.6%) TransTx | 12 (10.4%) TT 103 (89.6%) STT | 115 | 5.3 | |
| Wong et al. 67 | 72 (21 M, 51 F); 58.5 | 72 (100%) | 8 (11.1%) | “extending below the plane of the thoracic inlet on CT scan“ | 100% CT | 72 (100%) TransCx | 49 (68.1%) TT 23 (31.9%) HT | 120 | 2 | |
| CT: computed tomography; CXR: chest x-ray/radiograph; FNA: fine needle aspiration; HEM: haemorrhage; HT: hemithyroidectomy; MRI: magnetic resonance imaging; NA: not available; | ||||||||||
| PHC: permanent hypocalcaemia; PRLNP: permanent recurrent laryngeal nerve palsy; ITG: intrathoracic goiter; TransTx: sternotomy; STT: subtotal thyroidectomy; | ||||||||||
| TransCx: transcervical; TRACH: tracheomalacia; TT: total thyroidectomy, US: ultrasound. | ||||||||||
| Author | Overall number and % of postoperative complications | Haemorrhage | Permanent hypocalcaemia | Permanent recurrent laryngeal nerve palsy | Tracheomalacia | Other | Perioperative mortality (30-days) |
|---|---|---|---|---|---|---|---|
| Sahbaz et al. 44 | 0 (0%) | NA | 0 (0%) | 0 (0%) | NA | 0 (0%) | 0 (0%) |
| Tostado et al. 45 | 23 (38.3%) | NA | 0 (0%) | 1 (1.7%) | NA | 0 (0%) | 0 (0%) |
| Welman et al. 46 | 0 (0%) | 0 (0%) | 0 (0%) | NA | 0 (0%) | 0 (0%) | 0 (0%) |
| Cappellacci et al. 47 | 139 (3.1%) | 2 (0.7%) 28 (0.7%) | 9 (3.3%) 66 (1.6%) | 4 (1.4%) 17 (0.4%) | NA | 4 (1.4%) 9 (0.2%) Wound infection | 0 (0%) |
| Wang et al., 2022 48 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Tsur et al. 39 | 37 (14.1%) | 14 (5.2%) | 10 (3.8%) | 13 (4.9%) | NA | NA | 0 (0%) |
| Nakaya et al. 49 | 1 (2.3%) | 0 (0%) | 0 (0%) | 1 (2.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ghabisha et al. 50 | 4 (14.2%) | 2 (7.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Sulaiman et al. 51 | NA | NA | NA | NA | 3 (7.5%) | 2 (5%) Tracheostomy | 0 (0%) |
| Dagan et al. 52 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ren et al. 53 | NA | NA | NA | NA | 17 (16%) | NA | NA |
| Oukessou et al. 54 | 5 (4.3%) | 1 (0.8%) | 2 (1.7%) | 2 (1.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abdelrahman et al. 55 | 6 (20%) | 0 (0%) | 0 (0%) | 1 (3.4%) | 4 (13.4%) | 1 (3.4%) Pneumothorax | 0 (0%) |
| Aghajanzadeh et al. 56 | 33 (47.1%) | NA | NA | NA | NA | NA | 0 (0%) |
| Battistella et al. 57 | 3 (1.1%) | 2 (0.7%) | 0 (0%) | 0 (0%) | 1 (0.3%) | 0 (0%) | 1 (0.3%) |
| Chen et al. 58 | 11 (4.8%) | 6 (2.6%) | 1 (0.4%) | 4 (1.7%) | NA | 0 (0%) | 0 (0%) |
| Ching et al. 42 | 9 (19.5%) | 8 (17%) | 1 (2%) | 0 (0%) | NA | 0 (0%) | 0 (0%) |
| Damiano et al. 59 | 1 (1.7%) | NA | 1 (1.7%) | NA | NA | 0 (0%) | 0 (0%) |
| Doulaptsi et al. 43 | 2 (0.9%) | 0 (0%) | 0 (0%) | 2 (0.9%) | NA | 0 (0%) | 0 (0%) |
| Głód et al. 60 | NA | NA | NA | 20 (1.3%) | NA | NA | 0 (0%) |
| Li et al. 61 | 12 (8.4%) | 3 (2.1%) | 4 (2.8%) | 5 (3.5%) | NA | 0 (0%) | 0 (0%) |
| Linhares et al. 62 | 1 (0.9%) | 1 (0.9%) | 0 (0%) | 0 (0%) | NA | 0 (0%) | 0 (0%) |
| Tabchouri et al. 63 | 9 (12.8%) | 1 (1.4%) | 0 (0%) | 8 (11.7%) | NA | 0 (0%) | 0 (0%) |
| Van Slycke et al. 64 | 8 (8.4%) | 5 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3.1%) Pulmonary morbidity | 0 (0%) |
| Vaiman et al. 65 | 8 (11.4%) | NA | 2 (2.8%) | 1 (1.4%) | 5 (7.2%) | 0 (0%) | 0 (0%) |
| Wang et al. 66 | 4 (3.5%) | 0 (0%) | 0 (0%) | 1 (0.9%) | 2 (1.7%) | 1 (0.9%) Pleural effusion | 0 (0%) |
| Wong et al. 67 | 2 (2,8%) | 0 (0%) | 0 (0%) | 1 (1.4%) | 0 (0%) | 1 (1.4%) Tracheostomy | 0 (0%) |
| Mean | 14.3 | 3.6 | 4 | 3.5 | 2.2 | 0.8 | 0.04 |
| (n, %) | (3.7%) | (2.3%) | (0.1%) | (0.1%) | (1.7%) | (0.2%) | (0%) |
| NA: not available. | |||||||
References
- Gittoes N, Miller M, Daykin J. Upper airways obstruction in 153 consecutive patients presenting with thyroid enlargement. BMJ. 1996;312. doi:https://doi.org/10.1136/bmj.312.7029.484
- Nixon I, Simo R. The neoplastic goitre. Curr Opin Otolaryngol Head Neck Surg. 2013;21:143-149. doi:https://doi.org/10.1097/MOO.0b013e32835cec37
- Rugiu M, Piemonte M. Surgical approach to retrosternal goitre: do we still need sternotomy?. Acta Otorhinolaryngol Ital. 2009;29:331-338.
- Hanson M, Shaha A, Wu J. Surgical approach to the substernal goiter. Best Pract Res Clin Endocrinol Metab. 2019;33. doi:https://doi.org/10.1016/j.beem.2019.101312
- Simo R, Nixon I, Vander Poorten V. Surgical management of intrathoracic goitres. Eur Arch Otorhinolaryngol. 2019;276:305-314. doi:https://doi.org/10.1007/s00405-018-5213-z
- Page M, McKenzie J, Bossuyt P. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88. doi:https://doi.org/10.1016/j.ijsu.2021.105906
- Unlu M, Aygun N, Kostek M. Substernal goiter: from definitions to treatment. Sisli Etfal Hastan Tip Bul. 2022;56:167-176. doi:https://doi.org/10.14744/SEMB.2022.30806
- Rios A, Rodriguez J, Balsalobre M. The value of various definitions of substernal goiter for predicting intra-operative and postoperative complications. Surgery. 2010;147:233-238.
- Liddy W, Netterville J, Randolph G. Surgery of the Thyroid and Parathyroid Glands. (Liddy W, ed.). Elsevier; 2021.
- Zamora E, Ghandili S, Zamora M. Incidental primary intrathoracic goiter: dual-isotope scintigraphy and early-MIBI SPECT/CT. World J Nucl Med. 2022;21:148-151. doi:https://doi.org/10.1055/s-0042-1750337
- Regal M, Kamel M, Alyami H. Mediastinal ectopic thyroid mass with normal thyroid function and location: case report. Int J Surg Case Rep. 2018;52:5-7. doi:https://doi.org/10.1016/j.ijscr.2018.09.033
- Faroq Abdulrahman S, Teksöz S, Ferahman S. Missed thyroid gland after total thyroidectomy. Turk J Surg. 2018;34:137-139. doi:https://doi.org/10.5152/turkjsurg.2017.3206
- Oh S, Chia C, Ooi O. A rare case of ectopic retrosternal goiter. Clin Case Rep. 2021;9:1849-1852. doi:https://doi.org/10.1002/ccr3.3610
- Sohail A, Shahabuddin S, Siddiqui M. Ectopic thyroid mass separately present in mediastinum and not a retrosternal extension: a report of two cases. Case Rep Surg. 2019;2019. doi:https://doi.org/10.1155/2019/3821767
- Rico F, Lung J. Thyroid embryonic anomalies involving the medial and lateral anlagen: two surgical case reports. Case Rep Surg. 2019;2019. doi:https://doi.org/10.1155/2019/3174848
- Stumpf M, Marques A, Kluthcovsky A. Presternal goiter. J Coll Physicians Surg Pak. 2019;29:574-576. doi:https://doi.org/10.29271/jcpsp.2019.06.574
- Bakkar S, Hamdeh Q, Haddadin R. Retrosternal goiter masquerading as type II respiratory failure. A case report. Int J Surg Case Rep. 2022;94. doi:https://doi.org/10.1016/j.ijscr.2022.107104
- Kristensen M, Abrahamsen J, Thomsen H. Intrathoracic goiter visualized on iodine-123 and technetium-99m single-photon emission computed tomography/computed tomography. World J Nucl Med. 2021;20:377-378. doi:https://doi.org/10.4103/wjnm.wjnm_90_21
- Iriarte M, Morales E, Velásquez M. Giant intrathoracic goiter of atypical presentation: a case report. Clin Pathol. 2020;13. doi:https://doi.org/10.1177/2632010X20916741
- Kwak H, Banks K, Hsu D. Incidental paratracheal lymph node lung adenocarcinoma in a patient with goiter: a case report. AME Case Rep. 2022;6. doi:https://doi.org/10.21037/acr-22-23
- Bianchi D, Scamporlino A, Costantini M. A case of cervico-mediastinal paraganglioma mimicking an ectopic goiter. Int J Surg Case Rep. 2021;86. doi:https://doi.org/10.1016/j.ijscr.2021.106357
- Pattashanee S, Puri G, Kataria K. Comparison of modified rose method of thyroid palpation versus other methods for the detection of retrosternal and nodular goiter. J ASEAN Fed Endocr Soc. 2022;37:4-13. doi:https://doi.org/10.15605/jafes.037.01.02
- Ziai H, Lebo N, Kielar A. Can thyroid ultrasonography predict substernal extension or tracheal compression in goiters?. Can Assoc Radiol J. 2018;69:422-429. doi:https://doi.org/10.1016/j.carj.2018.07.007
- Knobel M. An overview of retrosternal goiter. J Endocrinol Invest. 2021;44:679-691. doi:https://doi.org/10.1007/s40618-020-01391-6
- Yano T, Okada T, Sato H. Preoperative evaluation of substernal goiter by computed tomography in the extended neck position. Case Rep Oncol. 2021;14:1353-1358. doi:https://doi.org/10.1159/000518532
- Uludag M, Kostek M, Unlu M. Surgical treatment of substernal goiter part 1: surgical indications, pre-operative, and peroperative preparation. Sisli Etfal Hastan Tip Bul. 2022;56:303-310. doi:https://doi.org/10.14744/SEMB.2022.52280
- Tikka T, Nixon I, Harrison-Phipps K. Predictors of the need for an extracervical approach to intrathoracic goitre. BJS Open. 2019;3:174-179. doi:https://doi.org/10.1002/bjs5.50123
- Sormaz İC, Uymaz D, İşcan A. The value of preoperative volumetric analysis by computerised tomography of retrosternal goiter to predict the need for an extra-cervical approach. Balkan Med J. 2018;35:36-42. doi:https://doi.org/10.4274/balkanmedj.2017.0161
- Cankar Dal H. Difficult airway management and emergency tracheostomy in a patient with giant goiter presenting with respiratory arrest: a case report. Exp Ther Med. 2022;24. doi:https://doi.org/10.3892/etm.2022.11426
- Osinaike B, Ogunsiji A, Joseph O. Challenging airway management in a patient with retrosternal goiter presenting in respiratory distress. Niger J Surg. 2021;27:66-70. doi:https://doi.org/10.4103/njs.NJS_58_19
- Sajid B, Rekha K. Airway management in patients with tracheal compression undergoing thyroidectomy: a retrospective analysis. Anesth Essays Res. 2017;11:110-116. doi:https://doi.org/10.4103/0259-1162.186608
- Olusomi B, Aliyu S, Babajide A. Goitre-related factors for predicting difficult intubation in patients scheduled for thyroidectomy in a resource-challenged health institution in north central Nigeria. Ethiop J Health Sci. 2018;28:169-176. doi:https://doi.org/10.4314/ejhs.v28i2.8
- Mahajan S, Sekar L, Kumar S. Airway management in a patient with retrosternal goiter: a context-sensitive airway management strategy. Indian J Otolaryngol Head Neck Surg. 2022;74:2214-2216. doi:https://doi.org/10.1007/s12070-020-02083-6
- Jeong Y, Jun I, Ha S. Extracorporeal membrane oxygenation for the anesthetic management of a patient with a massive intrathoracic goiter causing severe tracheal obstruction with positional symptoms: a case report. Medicine (Baltimore). 2019;98. doi:https://doi.org/10.1097/MD.0000000000017650
- Pan Y, Chen C, Yu L. Airway management of retrosternal goiters in 22 cases in a tertiary referral center. Ther Clin Risk Manag. 2020;16:1267-1273. doi:https://doi.org/10.2147/TCRM.S281709
- Tasche K, Dorneden A, Swift W. Airway management in substernal goiter surgery. Ann Otol Rhinol Laryngol. Published online 2021. doi:https://doi.org/10.1177/00034894211014794
- Joll C. The indications for surgical treatment in goitre. Postgrad Med J. 1932;8:262-265. doi:https://doi.org/10.1136/pgmj.8.81.262
- Bartsch K, Luster M, Buhr J. Indications for the surgical management of benign goiter in adults. Dtsch Arztebl Int. 2018;115:1-7. doi:https://doi.org/10.3238/arztebl.2018.0001
- Tsur N, Levi L, Frig O. Extended cervical approach for retrosternal multinodular goiter. Acta Otorhinolaryngol Ital. 2024;44:21-26. doi:https://doi.org/10.14639/0392-100X-N2746
- Aygun N, Celayir M, Isgor A. The effect of strap muscle transection on voice and swallowing changes after thyroidectomy in patients without laryngeal nerve injury. Ann R Coll Surg Engl. 2022;104:517-524. doi:https://doi.org/10.1308/rcsann.2021.0225
- Liddy W, Wu C, Dionigi G. Varied recurrent laryngeal nerve course is associated with increased risk of nerve dysfunction during thyroidectomy: results of the surgical anatomy of the recurrent laryngeal nerve in thyroid surgery study, an international multicenter prospective anatomic and electrophysiologic study of 1000 monitored nerves at risk from the International Neural Monitoring Study Group. Thyroid. 2021;31:1730-1740. doi:https://doi.org/10.1089/thy.2021.0155
- Ching H, Kahane J, Foggia M. Medial approach for the resection of goiters with suprahyoid, retropharyngeal, or substernal extension. World J Surg. 2018;42:1415-1423. doi:https://doi.org/10.1007/s00268-018-4576-z
- Doulaptsi M, Karatzanis A, Prokopakis E. Substernal goiter: treatment and challenges. Twenty-two years of experience in diagnosis and management of substernal goiters. Auris Nasus Larynx. 2019;46:246-251. doi:https://doi.org/10.1016/j.anl.2018.07.006
- Sahbaz N, Tutal F, Aksakal N. Cancer frequency in retrosternal goiter. Am Surg. 2017;83:1390-1393.
- Chávez Tostado K, Velázquez-Fernandez D, Chapa M. Substernal goiter: correlation between grade and surgical approach. Am Surg. 2018;84:262-266.
- Welman K, Heyes R, Dalal P. Surgical treatment of retrosternal goitre. Indian J Otolaryngol Head Neck Surg. 2017;69:345-350. doi:https://doi.org/10.1007/s12070-017-1151-0
- Cappellacci F, Canu G, Rossi L. Differences in surgical outcomes between cervical goiter and retrosternal goiter: an international, multicentric evaluation. Front Surg. 2024;11. doi:https://doi.org/10.3389/fsurg.2024.1341683
- Wang R, Li J, Jiang J. Modified subxiphoid approach for surgical resection of a retrosternal goiter. Front Surg. 2022;9. doi:https://doi.org/10.3389/fsurg.2022.923389
- Nakaya M, Ito A, Mori A. Surgical treatment of substernal goiter: an analysis of 44 cases. Auris Nasus Larynx. 2017;44:111-115. doi:https://doi.org/10.1016/j.anl.2016.02.016
- Ghabisha S, Ahmed F, Al-wageeh S. Management of retrosternal goiter in resource-limited settings: outcomes from 28 cases using cervical approach. Cureus. 15. doi:https://doi.org/10.7759/cureus.41288
- Sulaiman A, Lutfi A, Ikram M. Tracheomalacia after thyroidectomy for retrosternal goitres requiring sternotomy – a myth or reality?. Ann R Coll Surg Engl. 2021;103:504-507. doi:https://doi.org/10.1308/rcsann.2021.0014
- Dagan E, Kleid S. Obviating the need for sternotomy: safety and effectiveness of microdebrider use for retrosternal goiter. Head Neck. 2018;40:837-841. doi:https://doi.org/10.1002/hed.25070
- Ren W, Shang X, Fu H. Prolonged endotracheal intubation: a feasible option for tracheomalacia after retrosternal goitre surgery. Ann Palliat Med. 2020;9:1764-1769. doi:https://doi.org/10.21037/apm-19-552
- Oukessou Y, Mennouni M, Douimi L. Cervical approach to cervico-mediastinal goiters: experience of a Moroccan ENT tertiary center – Case series. Ann Med Surg (Lond). 2021;62:353-357. doi:https://doi.org/10.1016/j.amsu.2021.01.081
- Abdelrahman H, Al-Thani H, Al-Sulaiti M. Clinical presentation and surgical treatment of retrosternal goiter: a case series study. Qatar Med J. 2020;2020. doi:https://doi.org/10.5339/qmj.2020.13
- Aghajanzadeh M, Asgary M, Mohammadi F. An investigation into symptoms, diagnosis, treatment, and treatment complications in patients with retrosternal goiter. J Family Med Prim Care. 2018;7:224-229. doi:https://doi.org/10.4103/jfmpc.jfmpc_286_17
- Battistella E, Pomba L, Sidoti G. Retrosternal goitre: anatomical aspects and technical notes. Medicina (Kaunas). 2022;58. doi:https://doi.org/10.3390/medicina58030349
- Chen Q, Su A, Zou X. Clinicopathologic characteristics and outcomes of massive multinodular goiter: a retrospective cohort study. Front Endocrinol (Lausanne). 2022;13. doi:https://doi.org/10.3389/fendo.2022.850235
- Damiano G, Cocchiara G, Palumbo V. Iatrogenic hypoparathyroidism after surgery for retrosternal goitre. A single centre retrospective analysis. Clin Ter. 2018;169:E67-E70. doi:https://doi.org/10.7417/T.2018.2056
- Głód M, Marciniak D, Kaliszewski K. Analysis of risk factors for phonation disorders after thyroid surgery. Biomedicines. 2022;10. doi:https://doi.org/10.3390/biomedicines10092280
- Li W, Li H, Zhang S. To explore the risk factors and preventive measures affecting the treatment of retrosternal goiter: an observational study. Medicine (Baltimore). 2020;99. doi:https://doi.org/10.1097/MD.0000000000023003
- Linhares S, Scola W, Remer L. Morbidity associated with surgical removal of substernal thyroid goiters. J Surg Res. 2022;277:254-260. doi:https://doi.org/10.1016/j.jss.2022.04.018
- Tabchouri N, Anil Z, Marques F. Morbidity of total thyroidectomy for substernal goiter: a series of 70 patients. J Visc Surg. 2018;155:11-15. doi:https://doi.org/10.1016/j.jviscsurg.2017.05.006
- Van Slycke S, Simons A, Van Den Heede K. Combined cervicosternotomy and cervicotomy for true retrosternal goiters: a surgical cohort study. Updates Surg. 2021;73:1-10. doi:https://doi.org/10.1007/s13304-021-01027-1
- Vaiman M, Bekerman I, Basel J. Surgical approach to the intrathoracic goiter. Laryngoscope Investig Otolaryngol. 2018;3:127-132. doi:https://doi.org/10.1002/lio2.146
- Wang X, Zhou Y, Li C. Surgery for retrosternal goiter: cervical approach. Gland Surg. 2020;9:392-400. doi:https://doi.org/10.21037/gs.2020.03.43
- Wong W, Shetty S, Morton R. Management of retrosternal goiter: retrospective study of 72 patients at two secondary care centers. Auris Nasus Larynx. 2019;46:129-134. doi:https://doi.org/10.1016/j.anl.2018.06.012
- Chen Y, Kim H, Anuwong A. Transoral robotic thyroidectomy versus transoral endoscopic thyroidectomy: a propensity-score-matched analysis of surgical outcomes. Surg Endosc. 2021;35:6179-6189. doi:https://doi.org/10.1007/s00464-020-08114-1
- Martinez J, González M, Hernández Q. Goiter surgery recommendations in sub-Saharan Africa in humanitarian cooperation. Laryngoscope Investig Otolaryngol. 2022;7:417-424. doi:https://doi.org/10.1002/lio2.764
- Dessie G, Amare D, Dagnew A. Prevalence of goiter among children in Ethiopia and associated factors: a systematic review and meta-analysis. BMC Public Health. 2019;19. doi:https://doi.org/10.1186/s12889-019-7505-7
- Nakayama H, Goda M, Kohagura K. A large substernal goiter that extended to both sides of the thorax. Case Rep Surg. 2018;2018. doi:https://doi.org/10.1155/2018/6107982
- Ahuja K, Bhandari T, Banait-Deshmane S. Incidentally detected ectopic thyroid in juxta cardiac location-Imaging and pathology. Radiol Case Rep. 2018;13:909-913. doi:https://doi.org/10.1016/j.radcr.2018.06.004
- Kocaman G, Yenigün B, Çoruh A. Intrapericardial goiter. Gen Thorac Cardiovasc Surg. 2020;68:1051-1054. doi:https://doi.org/10.1007/s11748-019-01211-6
- Abdelhamid A, Aspinall S. Intraoperative nerve monitoring in thyroid surgery: analysis of United Kingdom registry of endocrine and thyroid surgery database. Br J Surg. 2021;108:182-187. doi:https://doi.org/10.1093/bjs/znaa081
- Khan N, Zhang Y, Bollig K. Extracervical approaches to substernal thyroid goiter resection: a systematic review and meta-analysis. OTO Open. 2024;8. doi:https://doi.org/10.1002/oto2.103
- Casella C, Molfino S, Cappelli C. Thyroiditis process as a predictive factor of sternotomy in the treatment of cervico-mediastinal goiter. BMC Surg. 2019;18. doi:https://doi.org/10.1186/s12893-019-0474-z
- Linhares S, Scola W, Remer L. Depth of mediastinal extension can predict sternotomy need for substernal thyroid goiters. Surgery. 2022;172:1373-1378. doi:https://doi.org/10.1016/j.surg.2022.06.026
- Haciyanli S, Karaisli S, Acar N. Split sternotomy in retrosternal thyroid and mediastinal parathyroid pathologies. Sisli Etfal Hastan Tip Bul. 2021;55:318-324. doi:https://doi.org/10.14744/SEMB.2021.76401
- Abdullah A, Bahjat A, Mohammed A. Huge toxic goiter extending to the posterior mediastinum: case report with literature review. Int J Surg Case Rep. 2019;62:69-72. doi:https://doi.org/10.1016/j.ijscr.2019.08.016
- Machboua A, Thumerel M, Hustache-Castaing R. Cervicotomy using a hemi-clamshell approach for a rare enlarged substernal goitre. Interact Cardiovasc Thorac Surg. 2022;35. doi:https://doi.org/10.1093/icvts/ivac056
- Nesti C, Wohlfarth B, Borbély Y. Case report: modified thoracoscopic-assisted cervical resection for retrosternal goiter. Front Surg. 2021;8. doi:https://doi.org/10.3389/fsurg.2021.695963
- Nakamura R, Okuda K, Chiba K. A large intrathoracic goiter with tracheal stenosis: complete resection using a robot-assisted thoracoscopic approach. Thorac Cancer. 2022;13:1874-1877. doi:https://doi.org/10.1111/1759-7714.14470
- Amore D, Cicalese M, Scaramuzzi R. Antero mediastinal retrosternal goiter: surgical excision by combined cervical and hybrid robot-assisted approach. J Thorac Dis. 2018;10:E199-E202. doi:https://doi.org/10.21037/jtd.2018.01.169
- Wadhavkar N, Kontopidis I, Bollig C. Transcervical and robotic-assisted thoracoscopic resection of a substernal goiter. BMJ Case Rep. 2022;15. doi:https://doi.org/10.1136/bcr-2022-250953
- Saito Y, Ikeda Y, Takami H. Combined thoracoscopic and axillary subcutaneous endoscopic thyroidectomy: a novel approach for cervicomediastinal goiters. Langenbecks Arch Surg. 2022;407:2169-2175. doi:https://doi.org/10.1007/s00423-022-02579-5
- Crudeli C, Reid L, Burg J. Minimally invasive combined approach robotic thymectomy and transcervical total thyroidectomy. BMJ Case Rep. 2022;15. doi:https://doi.org/10.1136/bcr-2022-249292
- Karsan R, Nanjaiah P, Hogan J. Concomitant aortic valve replacement and retrosternal goitre resection via mini-sternotomy: a novel case. J Surg Case Rep. 2020;2020. doi:https://doi.org/10.1093/jscr/rjaa066
- Piazza C, Duranti L, Giannini L. The transmanubrial osteomuscular-sparing approach: a valuable adjunct to the head and neck surgical armamentarium. Curr Opin Otolaryngol Head Neck Surg. 2020;28:61-67. doi:https://doi.org/10.1097/MOO.0000000000000605
- Testini M, Gurrado A, Avenia N. Does mediastinal extension of the goiter increase morbidity of total thyroidectomy? A multicenter study of 19,662 patients. Ann Surg Oncol. 2011;18:2251-2259. doi:https://doi.org/10.1245/s10434-011-1596-4
- Fiorelli R, Duarte A, de Quadros Teixeira A. Anatomical and developmental aspects of iatrogenic injury to the right recurrent laryngeal nerve in surgical resections of substernal goiter. Anat Rec (Hoboken). 2021;304:1242-1254. doi:https://doi.org/10.1002/ar.24629
- Tausanovic K, Zivaljevic V, Grujicic S. Case control study of risk factors for occurrence of postoperative hematoma after thyroid surgery: ten year analysis of 6938 operations in a tertiary center in Serbia. World J Surg. 2022;46:2416-2422. doi:https://doi.org/10.1007/s00268-022-06634-6
- de Carvalho A, Gomes C, Chulam T. Risk factors and outcomes of postoperative neck hematomas: an analysis of 5,900 thyroidectomies performed at a Cancer Center. Int Arch Otorhinolaryngol. 2021;25:E421-E427. doi:https://doi.org/10.1055/s-0040-1714129
- Doran H, Wiseman S, Palazzo F. Post-thyroidectomy bleeding: analysis of risk factors from a national registry. Br J Surg. 2021;108:851-857. doi:https://doi.org/10.1093/bjs/znab015
- Celik S, Konca C, Genc V. A cohort study assessing the association between body composition parameters and symptomatic hypocalcemia after total thyroidectomy. Am Surg. 2021;87:1305-1312. doi:https://doi.org/10.1177/0003134820979578
- Chen Z, Zhao Q, Du J. Risk factors for postoperative hypocalcaemia after thyroidectomy: a systematic review and meta-analysis. J Int Med Res. 2021;49. doi:https://doi.org/10.1177/0300060521996911
- Zuo T, Gao Z, Chen Z. Surgical management of 48 patients with retrosternal goiter and tracheal stenosis: a retrospective clinical study from a single surgical center. Med Sci Monit. 2022;28. doi:https://doi.org/10.12659/MSM.936637
- Hsu S, Melucci A, Dave Y. Outpatient endocrine surgery practice patterns are highly variable among US endocrine surgery fellowship programs. Surgery. 2023;173:76-83. doi:https://doi.org/10.1016/j.surg.2022.05.004
- Jeannon J, Orabi A, Bruch G. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract. 2009;63:624-629. doi:https://doi.org/10.1111/j.1742-1241.2008.01875.x
- Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. doi:https://doi.org/10.1097/01.sla.0000133083.54934.ae
- Bukarica S, Antić J, Fratrić I. Thyroid surgery in children: a 5-year retrospective study at a single Paediatric Surgical Center and systematic review. Children (Basel). 2022;9. doi:https://doi.org/10.3390/children9121818
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1694 times
- PDF downloaded - 221 times




