Head and neck
Vol. 45: Issue 3 - June 2025
Exploring the use of submental flap for parotid region reconstruction: a multicentric experience.
Abstract
Objectives. This study aims to evaluate the effectiveness and reproducibility of the submental island flap (SIF) for reconstructing defects in the parotid region after oncological surgery. The primary goals are to assess the flap’s impact on patient morbidity, its overall feasibility in complex head and neck reconstructions, and its potential for consistent outcomes across different cases.
Methods. A retrospective multicentric study was conducted across 6 tertiary centres in Northern Italy, reviewing cases from 2015 to 2023. Inclusion criteria encompassed adult patients undergoing parotid region reconstruction using the SIF, specifically for defects arising from parotid or associated skin tumours. Data on patient demographics, comorbidities, tumour characteristics, surgical
details, flap characteristics, complications, and long-term oncological outcomes were collected and analysed.
Results. The study included 30 patients with a mean age of 75.4 years, most of whom had significant comorbidities. The flap success rate was 93.3%, with minimal donor site morbidity. The aesthetic outcomes were favourable, with the flap providing a good match of colour and texture. Oncological safety was affirmed, with no nodal transfers observed.
Conclusions. The SIF is a reliable and aesthetically favourable option for parotid region reconstruction, particularly in elderly patients with multiple comorbidities. Its use minimises donor site morbidity and does not require microsurgical expertise. Careful patient selection is critical to avoid complications, particularly in those with a history of submental trauma, and to not risk metastatic lymph node transfer in advanced nodal disease. The SIF allows for effective reconstruction without compromising oncological outcomes, supporting its use as a standard approach in appropriate cases.
Introduction
Reconstruction of the parotid region following oncological surgery may be needed in case of primary skin cancer or skin invasion from parotid tumours or metastasis to periparotid or intraparotid lymph nodes 1. Besides the possible reconstruction of the facial nerve, there is no specific function to restore, and therefore the main reconstructive goal to achieve is closure of the defect with recovery of the aesthetic appearance of a highly visible region of the face, restoring facial contour and symmetry and optimising colour and texture matching of the skin.
Various options are available according to the extent of the defect. Smaller defects can usually be closed by primary suture or with local skin flaps, thanks to the relative laxity of the skin in this region; however, in case of larger defects or previous surgery or radiotherapy, locoregional pedicled flaps or free flaps are required 2.
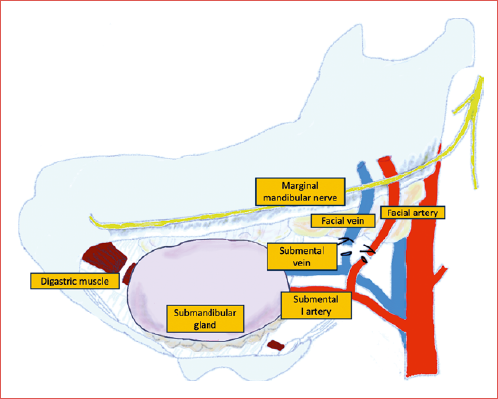
The submental island flap (SIF) has recently emerged as a valuable option for reconstruction of many head and neck defects and has gained popularity thanks to its versatility and reliability. The SIF is pedicled on the submental artery, a constant branch of the facial artery, and its vein (Cover figure), and allows the transfer of soft tissues from the chin, including up to 18 x 7 cm of skin, together with fascia and muscle 3. Its skin perfectly matches the colour and texture of the parotid region and in men it also bears beard hairs that help cosmetic camouflage. It provides the advantage of being harvested from the same surgical field with minimal to no donor site morbidity. However, potential risks include submental skin dehiscence and aesthetic concerns, especially in thin patients 4.
Furthermore, its use does not require microsurgical expertise and longer surgical time related to free flap reconstruction, which proves particularly useful considering that skin cancer mostly affects elderly patients who are therefore more prone to have multiple comorbidities and potential complications.
Despite its many advantages, few reports are present in the literature reporting the application of the SIF for reconstruction of the parotid region. The present study seeks to address this gap, offering a retrospective multicentric analysis of patient outcomes following parotid region reconstruction with the SIF, with the aim to demonstrate its reliability, reproducibility and optimal aesthetic results without compromising oncological outcomes, thanks to the particular importance given to the selection criteria of patients.
Methods
A retrospective study was conducted in 6 tertiary centres in Northern Italy. Cases from 2015 to 2023 were reviewed, including all patients who underwent reconstruction with a submental island flap for defects in the parotid region. The study was approved by the Institutional Ethics Committee of Bolzano (59-2024).
The inclusion criteria encompassed patients affected by parotid tumours of any histology or skin tumours of the parotid region, whether or not these were associated with extended parotidectomy, provided that reconstruction was performed using a submental island flap. Patients had to have a preoperative clinical N0 or N+ neck without evidence of metastasis at level I. Additionally, only adult patients aged 18 years and older were eligible for inclusion. The exclusion criteria were patients with defects in the parotid region reconstructed with a submental flap for non-oncological reasons, such as trauma or benign neoplasms. Also excluded were patients who underwent reconstruction using free flaps or pedicled flaps other than the submental flap.
Data regarding patient demographics, comorbidities, tumour characteristics, and surgical defect details were retrospectively collected, along with information on the surgical procedure, the anatomical structures of the parotid region that were preserved or sacrificed, and the size of the surgical defect. The characteristics of the reconstructive submental flap were also reported (inclusion of the anterior belly of the digastric muscle, mylohyoid muscle or submandibular gland). Subsequently, data on flap survival, complications, nodal transfer, and long-term oncological outcomes were evaluated.
Flap harvesting, surgical technique
Flap anatomy: the SIF is supplied by the submental artery. The submental artery is the biggest branch of the facial artery. It originates from the facial artery after it exits from the submandibular gland, about 30 mm from its origin and 5 mm from the mandibular border. The diameter of the submental artery ranges between 1 and 1.7 mm at the origin. The submental artery courses medially between the submandibular gland and the pre-glandular lymph nodes and then runs over the mylohyoid muscle beneath the mandibular border. The submental artery runs deep to the anterior belly of the digastric muscle in 70-81% of cases. Along its path, it gives rise to 1-4 cutaneous perforators that penetrate the platysma muscle, forming a subdermal plexus that extensively anastomoses with branches from the opposite side in more than 90% of cases. There are usually 2 perforators, one laterally and one medially to the anterior belly of the digastric muscle and minor perforators that run through the muscle itself. As the artery reaches the chin, it turns upward at the mandibular symphysis, crossing over it before splitting into 2 terminal branches that supply the chin and lower lip. Venous drainage of the flap occurs through the submental vein into the facial vein, with 1-3 anastomoses connecting to the external jugular vein 5.
Flap design: the size of the harvestable flap varies significantly from patient to patient. This depends on both the submental fat pad, which is more developed in overweight patients and less so in thin ones, and the skin laxity of the submental region, which is generally more pliable in elderly patients. Preoperatively, it is useful to perform a pinch test on the submental skin to evaluate the amount of skin that can be harvested and still allow primary closure of the donor site defect. The flap is designed with an oval shape centered on the submental region, with the superior incision approximately 1 cm below the inferior margin of the mandibular symphysis to hide the scar as much as possible and to prevent inferior lip eversion. The inferior margin is placed in a skin fold at the supra-hyoid level, at a distance previously assessed with the pinch test and based on the cutaneous defect to be reconstructed. Posteriorly, the incision continues in the neck along the incision made for parotidectomy, with or without neck dissection.
Flap dissection: the flap dissection begins on the side opposite to the pedicle, along the inferior margin, lifting the skin paddle in the subplatysmal plane. It is useful to identify the hyoid bone and the intermediate tendon of the digastric muscle bilaterally as landmarks, identify the mylohyoid muscle plane, and superiorly isolate the insertion of the anterior belly of the digastric muscle at the mandible. The dissection should proceed above the plane of the mylohyoid muscle using blunt dissection and preferably cold instruments to avoid damaging any cutaneous perforators of the flap, until reaching the anterior belly of the ipsilateral digastric muscle. At this point, the submental vessels are identified at the medial border of the anterior belly of the digastric muscle. The anterior belly of the digastric muscle is then detached from the mandibular insertion and divided at the intermediate tendon and included in the flap to protect the vascular pedicle and to minimise the risk of injuring the major perforators that run along both sides of the muscle.
Dissection of the vascular pedicle: the facial artery and vein are identified and then dissected up to their origin from the external carotid artery and thyro-lingual-facial trunk. The vessels are then ligated above the mandibular margin. The surgeon must take special care not to damage the marginal mandibular branch of the facial nerve at this step. The submental artery and vein are identified at their origin from the facial artery. In cases where a level IB lymph node dissection is indicated, the submental vessels are dissected, ligating all branches leading to the submandibular gland. The dissection and skeletonisation of the pedicle should be stopped once a vascular pedicle of sufficient length to reach the defect is obtained. If necessary, the pedicle length can be increased by additional dissection. The division of the facial vessels distal to the origin of the submental artery provides an additional 1-2 cm of pedicle length.
Donor site closure: the flap can then be harvested and transferred to the defect site. The donor site is closed by primary intention.
Figure 1 illustrates the surgical procedure for an extended parotidectomy and subsequent reconstruction using a submental flap in a patient with a retroauricular skin squamous cell carcinoma (SCC).
Figure 2 presents the surgical procedure for a patient diagnosed with mucoepidermoid carcinoma of the parotid gland with skin infiltration, who underwent an extended parotidectomy, neck dissection, and reconstruction using a submental flap.
Surgical considerations
Management of the submental artery: skeletonisation of the submental pedicle with ligation of the vessels directed toward the submandibular gland is necessary in cases of SCC with potential metastasis to level IB, and in all cases of a cN+ neck. In some selected cases involving patients with non-SCC parotid tumours, where a level I dissection would not be indicated, it is possible to avoid dissection of the pedicle at this level by skeletonising only the facial artery and preserving the fatty tissue of level IB and the submandibular gland within the flap, thus avoiding dissection of the proximal part of the submental artery. This approach reduces the risk of injuring the artery itself, allows for increased flap volume, and saves time during flap preparation. The essential prerequisite is that there is no risk of pathological nodal transfer, which would contraindicate this approach in other reconstruction sites such as the oral cavity, but it may be possible for selected cases of non-lymphotropic parotid tumours. This strategy was applied in 2 out of 6 centres where it was indicated. The length of the arterial pedicle is usually sufficient to reach the parotid region to be reconstructed. If additional length is needed, dissection of the entire facial pedicle facilitates rotation and distal transposition of the flap.
Management of venous drainage: in most cases, the length of the vascular pedicle is sufficient to reach the parotid region. However, in cases where the defect extends more cranially into the temporal or frontal region, the limiting factor for pedicle length is often the vein’s length. Certain techniques can be employed to increase the pedicle’s length in these situations. One strategy involves the Y-V procedure. A communicating branch between the facial vein and the external jugular vein is isolated, and the trunk of the facial vein is divided proximally to this communicating branch, allowing up to 5 cm of distal mobilisation of the flap. In this case, venous drainage occurs through the external jugular vein. For defects with a very cranial extension, one option can be to sever the submental or facial veins and anastomose them to veins near the recipient site 6. The venous pedicle elongation technique was used as an option in one of the 6 centres.
Soft tissue management: one of the main limitations of the submental flap is the reduced volume in patients with a thin adipose layer, especially when the surgical defect extends beyond the skin to the surrounding structures, leading to the loss of a large volume of soft tissues. The volume of the flap can be increased by including the anterior belly of the digastric muscle bilaterally, the mylohyoid muscle, and, in cases of non-SCC primary parotid tumours with cN0 neck, also the fat from level IB and the submandibular gland. In this case, it is advisable to cut the Wharton’s duct and include the gland in the flap. This strategy was used in 2 of 6 centres.
Surgical tips and tricks
Minimising risks to the marginal mandibular nerve: it is advisable to consider the use of intraoperative nerve monitoring (IONM) to help minimise the risk to the marginal mandibular nerve. Ideally, both sides of the nerve should be monitored, as the contralateral side may also be at risk, particularly in elderly patients where the nerve may be positioned lower. However, depending on the specific patient or situation, other strategies may also be effective.
Ensuring adequate venous drainage: venous drainage may not always be consistent, as demonstrated by cases of flap failure due to insufficient drainage. One approach could be preparing the flap before performing the neck dissection, which allows for better understanding of the venous anatomy and reduces the risk of ligating important vessels. If level IB removal is necessary, beginning the dissection there might be a prudent choice. Early commitment to the use of the submental flap is suggested to adjust the incision accordingly.
Including the mylohyoid muscle: some authors recommend routinely including the ipsilateral mylohyoid muscle in the flap and dissecting along the midline raphe. There is literature 3 supporting the immediate inclusion of the mylohyoid muscle. This avoids the need to dissect the pedicle in its thinner sections. If additional volume is required, the dissection can be extended beyond the midline raphe to include part of the contralateral mylohyoid muscle. However, depending on the situation, the inclusion of the mylohyoid muscle may be adapted or reconsidered based on specific case requirements.
Results
From the retrospective analysis performed, a total of 30 patients who met the inclusion criteria were included. Among these, 6 were females and 24 were males. The mean age at the time of surgery was 75.4 years with a median of 78 (range, 43-86). The majority of patients (80%) had at least one comorbidity, with 40% having 2 or more (Tab. I). Out of the total, 8 patients had previously undergone a cutaneous resection in the parotid region, while 3 patients underwent superficial parotidectomy. Only 2 patients received radiotherapy in the head and neck region prior to surgery and one chemotherapy. All patients underwent skin resection in the parotid region, with almost all cases being associated with extended parotidectomy (27/30 patients). The details of the procedure and the resected anatomical structures are summarised in Table II, while Table 3 summarises tumour staging and follow-up data. The mean defect size was 43.8 cm2 with a median of 42 cm2 (range, 12-105 cm2). Figure 3 shows the distribution of skin defects among the different centres, indicating a substantial uniformity in the indications for reconstruction using the submandibular flap. The size of the flap harvested was (a-1) x (b-1), where a and b are the width and height of the defect, respectively. In 14 cases, the anterior belly of the contralateral digastric muscle was also harvested with the submental flap, in 8 the mylohyoid muscle, and in 4 the submandibular gland. Regarding flap-related complications, we observed 2 total flap necroses due to venous stasis, one of which occurred in a patient with a scar due to a submental trauma happened approximately 10 years before. These 2 patients were managed in one case with debridement and replacement with a pedicled pectoralis major flap, and in the other with debridement and healing by secondary intention. We also observed 2 patients with cutaneous venous stasis that resolved with conservative therapy. After these considerations, the flap success rate was 93.3%. Additional postoperative complications included 2 delayed wound healings at the level of the external auditory canal and 1 bleeding requiring surgical revision, as well as 2 medical complications: delirium and urinary tract infection. The mean follow-up was 39.2 months (range, 6-120). At the last follow-up, 20 of 30 patients were alive without disease (NED), 7 were alive with disease (AWD), one died of disease (DOD), and 2 died of other causes (DOC). Recurrence occurred in 4 patients locally (one in the external auricular canal and 3 on the preauricular skin adjacent to the submental flap). Although these local recurrences raise concerns that the use of SIF may limit the ability to extend the skin resection within safe oncologic margins, it is important to note that all recurrences occurred outside the reconstructed area. Furthermore, none of these patients had close or positive margins on definitive histology. However, the theoretical advantage of free flaps in providing wider coverage remains a consideration, particularly in cases requiring extended skin resection. Locoregional recurrence was observed, involving level III. Although clearance of IA-IB-IIA levels may theoretically be hindered by SIF harvesting, all patients in our series underwent meticulous lymph nodes dissection before flap elevation, ensuring oncological safety. Importantly, we observed no pathological nodal transfer with flap transposition, further supporting the safety of the technique when applied with proper patient selection and oncological principles in mind.
Figure 3 shows the distribution of the size of the skin defect between the centres.
Discussion
Surgical defects in the parotid region present unique reconstructive challenges, including contour restoration, skin coverage and facial reanimation. Furthermore, given the aggressive nature of malignancies necessitating this type of resection, the reconstruction must be robust and reliable to facilitate the timely initiation of postoperative adjuvant radiotherapy 1.
The reconstructive options primarily depend on several key factors:
- the defect’s size includes both the extent of skin removal and the underlying volume needing reconstruction 7;
- lymph nodes status (especially given the risk of nodal transfer when using a submental flap). Additionally, it is important to acknowledge the risk of inadequate clearance of levels IA, IB, and IIA, which may leave occult nodal metastases;
- the patient’s overall health and medical condition 8;
- availability of suitable donor sites9;
- aesthetic and functional goals of the reconstruction 9;
- expertise and experience of the surgical centre performing the procedure 9;
- necessity of adjuvant treatments after surgery 10.
In our case series, we aimed to evaluate the effectiveness of the submental pedicled flap to reconstruct this type of defect, evaluating its indications and results in 6 different tertiary centres in Northern Italy.
The submental flap provided excellent aesthetic outcomes due to its colour match with facial skin and the presence of hair follicles in men, aligning well with the beard area. This makes it ideal for achieving a cosmetically pleasing result 11. Furthermore, the donor site morbidity associated with the submental pedicled flap is minimal, as shown by our results, which showed a morbidity rate of 0%. In fact, the submental flap donor site is within the same surgical field, allowing for the extension of the incision used for neck dissection, commonly performed in these patients. This surgical convenience adds to the feasibility of the procedure.
Additionally, it is important to highlight that the submental flap closely matches the shape and size of the parotid gland, with a thinner distal portion and a thicker proximal portion. The volume of the submental flap can be adjusted to suit the defect’s size by incorporating the mylohyoid muscle along with the anterior belly of the digastric muscle during the harvest. The flap’s size can be highly variable, depending on the laxity of the submental skin, providing flexibility in reconstructing defects of different dimensions 12.
The average age of the patients in our study, and generally those with cutaneous SCC metastasised to the parotid, tends to be advanced. These patients often have a significant history of sun exposure and multiple prior excisions of cutaneous SCCs on the head 13. Over time, they frequently develop parotid metastases, resulting in a population marked by increased frailty and numerous comorbidities. This is reflected in our study, where most patients had at least one comorbidity, and their ACE-27 scores ranged from 2 to 3, indicating moderate to severe comorbid conditions.
While reconstructive surgery with free flaps is not entirely contraindicated in this patient population 14-16, certain considerations are essential. One of the factors to consider include the extended duration of surgery required for free flaps procedures. Although this does not impact flap survival, prolonged anaesthesia in frail patients can lead to poorer outcomes and a higher risk of intra-operative and postoperative complications 17. Additionally, hospital stays tend to be longer 18. Furthermore, the availability of a microsurgeon at the centre is crucial for performing these procedures. In contrast to free flaps, the submental flap significantly reduces the duration of surgery and can be performed even in centers without a reconstructive microsurgical team.
However, there are challenges associated with the submental flap, particularly related to venous congestion. The venous anatomy of this region can vary, affecting the success of the procedure and the risk of complications, such as venous congestion and necrosis 19.
Regarding the use of indocyanine green (ICG) in assessing vascularisation, this dye is primarily used to evaluate blood flow, including both arterial and venous circulation. ICG angiography helps in visualising the perfusion of the flap, providing real-time information on both the arterial supply and venous drainage. This technique is particularly useful in assessing venous return, as it can highlight areas of venous congestion 20.
In our experience, flaps have failed due to venous congestion, with one notable case involving a patient with a previous submental trauma and a 5 cm scar. Despite evaluating the vascularisation with ICG, venous compromise still occurred. This underscores the importance of contraindicating the use of the submental flap in patients with a history of submental trauma.
Although this did not occur in our patients, the potential pathological nodal transfer from the donor site to the recipient site is another concern. The risk lies in the potential transfer of metastatic tissue to the recipient area or the possibility of cancer recurrence at the flap base. Chow et al. advised that dissecting in the subplatysmal plane could reduce the risk of tumour spread and ensure adequate clearance 21. Amin et al. recommended complete lymph nodes dissection before harvesting the flap and suggested avoiding this flap in patients with clinically advanced nodal disease in the neck 22.
For the aforementioned reasons, care should be taken in patients with neck metastasis or a history of neck dissection because the integrity of the facial artery and vein is crucial for the technique’s success. Utilising colour Doppler ultrasound to locate the facial artery/vein and skin perforators can significantly lower the failure rate 23,24. Regarding the presence of neck metastasis, this consideration is particularly relevant for cases using the submental flap for oral cavity reconstruction. Parotid tumours rarely metastasise to level IB lymph nodes, so if the clinical assessment indicates no involvement in level I, the use of the submental flap is safe. This has been supported by our results showing no cases of nodal transfer.
The authors believe that the concern about using the submental flap for parotid reconstruction, particularly regarding the risk of nodal transfer, is often cited but may not be as significant in this context. The submental flap can be safely utilised during level IB dissection if the integrity of the pedicle is maintained, ensuring effective reconstruction without compromising the procedure’s safety. Additionally, tumours in the parotid gland, except those located at the tail, rarely metastasise to level I nodes. Similarly, primary cutaneous tumours typically do not spread to this level, reducing concerns about nodal involvement. Furthermore, careful patient selection further mitigates risks, as this flap is generally avoided in patients with clinically positive nodes in level I (cN+). In our case series, none of the patients had pathological positive nodes (pN+) in level I. These outcomes support the safety and efficacy of the submental flap in appropriately selected cases, demonstrating its viability as a reconstructive option.
Further consideration should be given to nodal status as an important factor in decision-making. Given the complexity and risks associated with operating on patients with a clinical N3b status, it would be prudent to consider adding cN3b as a relative contraindication. While we successfully treated 6 patients with pN3b, in cases with preoperative cN3b, careful evaluation is essential due to the potential need to ligate significant vessels like the internal jugular vein or the thyrolinguofacial trunk during the procedure. This approach allows us to justify our past interventions while highlighting the need for caution in similar future cases.
Our study observed consistent results and indications across various centres, suggesting good standardisation and reproducibility of the procedure. Different surgeons from multiple centres provided similar recommendations, further supporting the reliability of the submental flap technique.
In conclusion, given the typical profile of patients with parotid SCC (elderly with significant comorbidities), the excellent aesthetic outcomes, ease of harvest, and low donor site morbidity, we advocate for the submental flap as a feasible option for reconstructing medium-sized defects (40-80 cm2) in the parotid region. This indication can be extended to smaller defects that cannot be closed primarily due to previous resections or to larger defects if there is sufficient submental skin laxity. The submental flap represents a robust, aesthetically favourable, and low-morbidity option for parotid region reconstruction in this vulnerable patient population.
However, careful patient selection is crucial, especially concerning those with a history of submental trauma or advanced nodal disease. Advanced imaging techniques, such as colour Doppler ultrasound, can enhance the success rate by ensuring adequate vascular supply.
Conclusions
The SIF is a reliable, reproducible, and aesthetically favourable option for parotid region reconstruction. Our multicentric study demonstrated a high success rate with minimal donor site morbidity, making it particularly suitable for elderly patients with comorbidities. Careful patient selection minimises risks such as venous congestion and nodal transfer. When appropriately used, it provides oncological safety and excellent aesthetic outcomes and avoids the need for microsurgical reconstruction. Given its function, aesthetics, and safety advantages, we advocate for the SIF as a first-line reconstructive option for parotid region defects in well-selected cases, offering a balance between oncological safety and optimal surgical outcomes.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
LG, DDS, SB: conceptualised the study, contributed to the study design, and participated in data interpretation and manuscript drafting; LG, DDS, SB, EZ, MT, RDB, GS, AG, AT, LVC: assisted in data acquisition, performed the statistical analysis, and contributed to the interpretation of the results; GM, LC, LG, LS, SF, MA, SBO: provided critical revisions and contributed to the final approval of the manuscript; LG, GM, LC, DDS, SB, EZ, MT, RDB, GS, AG, AT, LVC: played a role in the surgical procedures, contributed to data collection, and assisted in drafting the manuscript.
All authors have read and approved the final manuscript.
Ethical consideration
This study was approved by the Institutional Ethics Committee (Comitato etico per la sperimentazione clinica della provincia autonoma di Bolzano, 59-2024).
The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: November 7, 2024
Accepted: March 3, 2025
Figures and tables
Figure 1. Extended parotidectomy and reconstruction with submental flap. A) Preoperative view showing the retroauricular lesion with the planned design of the submental flap marked on the skin; B) The incision for the submental flap is outlined, demonstrating the planning required to ensure adequate tissue harvest while maintaining a vascular pedicle. The flap design is strategically placed to maximize the available skin and soft tissue for the reconstruction; C) Intraoperative image depicting harvesting of the submental flap. The flap is being elevated with attention to preserving the vascular supply; D) A detailed view of the vascular anatomy of the flap was taken after the completion of the parotidectomy. The image shows the submental artery and vein; E) The final postoperative outcome is shown, with the submental flap securely placed and the incisions closed. The result demonstrates good contour restoration and the successful coverage of the defect created by the extended parotidectomy.
Figure 2. Extendend parotidectomy, neck dissection and reconstruction with submental flap. A) Preoperative view of the patient, displaying the tumour’s infiltration of the skin; B) This image illustrates the surgical field following the extended parotidectomy and neck dissection. The extent of the resection, including removing the infiltrated skin and underlying tissue, is evident; C) Intraoperative image showing the harvested submental flap; D) Postoperative result demonstrates a successful outcome with good contour restoration, minimal scarring and excellent skin colour match.
Figure 3. Size of the skin defect (cm2): distribution among the 6 centres.
| Comorbidities | |
|---|---|
| Chronic renal failure | 6 (20%) |
| Cardiac issues | 9 (30%) |
| Hypertension | 6 (20%) |
| Diabetes | 8 (26.7%) |
| Hepatic cirrhosis | 3 (10%) |
| Obesity | 1 (3.3%) |
| Leukaemia | 1 (3.3%) |
| None | 5 (16.7%) |
| Number of comorbidities per patient | |
| 0 | 6 (20%) |
| 1 | 12 (40%) |
| 2 | 9 (30%) |
| 3 | 3 (10%) |
| ACE-27 score | |
| 0 | 6 (20%) |
| 1 | 1 (3.3%) |
| 2 | 13 (43.3%) |
| 3 | 10 (33.3%) |
| Surgical defect | |
|---|---|
| Skin resection and extended parotidectomy | 26 (86.7%) |
| External auricular canal | 7 (23.3%) |
| Masseter muscle | 3 (10%) |
| Petrosectomy | 2 (6.7%) |
| Sternocleidomastoid muscle | 5 (16.6%) |
| Temoporomandibular joint | 1 (3.3%) |
| Zygomatic arch | 1 (3.3%) |
| Facial nerve trunk | 1 (3.3%) |
| Marginal branches | 10 (33.3%) |
| Only skin resection | 3 (10%) |
| Skin resection + total petrosectomy | 1 (3.3%) |
| Treatment of the ipsilateral neck | |
| MRND | 6 (20%) |
| Comprehensive ND Level I-V | 11 (23.3%) |
| SND Level I-IV | 3 (10%) |
| SND Level II-V | 4 (13.3%) |
| SND Level IB-IV | 1 (3.3%) |
| SND Level II-III | 1 (3.3%) |
| Not available | 4 (13.3%) |
| MRND: modified radical neck dissection; SND: selective neck dissection; ND: neck dissection. | |
| Histological examination | |
|---|---|
| Squamous cell carcinoma | 22 (73.3%) |
| Basal cell carcinoma | 3 (10%) |
| Melanoma | 1 (3.3%) |
| Acinic cell carcinoma | 1 (3.3%) |
| Mucoepidermoid carcinoma | 1 (3.3%) |
| Carcinoma ex pleomorphic adenoma | 1 (3.3%) |
| Dermatofibrosarcoma protuberans | |
| 1 (3.3%) | |
| pT stage | |
| pTx | 3 (10%) |
| pT1 | 0 |
| pT2 | 5 (16.6%) |
| pT2a | 1 (3.3%) |
| pT3 | 8 (26.6%) |
| pT3c | 1 (3.3%) |
| pT4a | 11 (43.3%) |
| pT4b | 1 (3.3%) |
| pN stage | |
| pN0 | 17 (56.6%) |
| pN1 | 3 (10%) |
| pN2a | 2 (6.7%) |
| pN2b | 2 (6.7%) |
| pN3b | 6 (20%) |
| Adjuvant treatments | |
| None | 18 (60%) |
| RT | 10 (33.3%) |
| CRT | 1 (3.3%) |
| Immunotherapy | 1 (3.3%) |
| Follow-up status | |
| NED | 20 (66.7%) |
| AWD | 7 (23.3%) |
| DOD | 1 (3.3%) |
| DOD | 2 (6.7%) |
| RT: radiotherapy; CRT: chemoradiotherapy; NED: no evidence of disease; AWD: alive with disease; DOD: dead of disease; DOC: dead of other cause. | |
References
- Ch’ng S, Ashford B, Gao K. Reconstruction of post-radical parotidectomy defects. Plast Reconstr Surg. 2012;129:E275-E287. doi:https://doi.org/10.1097/PRS.0b013e318213a11a
- Jørgensen M, Tabatabaeifar S, Toyserkani N. Submental island flap versus free flap reconstruction for complex head and neck defects. Otolaryngol Head Neck Surg. 2019;161:946-953. doi:https://doi.org/10.1177/0194599819875416
- Patel U, Bayles S, Hayden R. The submental flap: a modified technique for resident training. Laryngoscope. 2007;117:186-189. doi:https://doi.org/10.1097/01.mlg.0000246519.77156.a4
- Li P, Li H, Ding S. Clinical application of submental island flaps in repair and reconstruction of head and neck tumors: retrospective review of a single-center experience. Ear Nose Throat J. Published online 2022. doi:https://doi.org/10.1177/01455613221136671
- Faltaous A, Yetman R. The submental artery flap: an anatomic study. Plast Reconstr Surg. 1996;97:56-62. doi:https://doi.org/10.1097/00006534-199601000-00008
- Ferrari S, Copelli C, Bianchi B. The submental island flap: pedicle elongation and indications in head and neck reconstruction. J Craniomaxillofac Surg. 2014;42:1005-1009. doi:https://doi.org/10.1016/j.jcms.2014.01.025
- Ragbir M, Brown J, Mehanna H. Reconstructive considerations in head and neck surgical oncology: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S191-S197. doi:https://doi.org/10.1017/S0022215116000621
- Ferrari S, Copelli C, Bianchi B. Free flaps in elderly patients: outcomes and complications in head and neck reconstruction after oncological resection. J Craniomaxillofac Surg. 2013;41:167-171. doi:https://doi.org/10.1016/j.jcms.2012.07.005
- Wei F, Mardini S. Flaps and Reconstructive Surgery. Elsevier; 2017.
- Bassani S, Eccher A, Molteni G. Harnessing the power of artificial intelligence: revolutionizing free flaps monitoring in head and neck tumor treatment. Crit Rev Oncog. 2023;28:25-30. doi:https://doi.org/10.1615/CritRevOncog.2023049158
- Dermody S, Kahng P, Rao V. Color match following free flap surgery in head and neck reconstruction: a colorimetric and aesthetic analysis. Plast Reconstr Surg. 2024;153:1142-1150. doi:https://doi.org/10.1097/PRS.0000000000010807
- Bayon R, Davis A. Submental flap for soft tissue reconstruction following radical parotidectomy. Otolaryngol Head Neck Surg. 2019;160:1130-1132. doi:https://doi.org/10.1177/0194599819827822
- Franzen A, Lieder A, Guenzel T. The heterogenicity of parotid gland squamous cell carcinoma: a study of 49 patients. In Vivo. 2019;33:2001-2006. doi:https://doi.org/10.21873/invivo.11696
- Sorg H, Sorg C, Tilkorn D. Free flaps for skin and soft tissue reconstruction in the elderly patient: indication or contraindication. Med Sci (Basel). 2023;11. doi:https://doi.org/10.3390/medsci11010012
- Grammatica A, Piazza C, Paderno A. Free flaps in head and neck reconstruction after oncologic surgery: expected outcomes in the elderly. Otolaryngol Head Neck Surg. 2015;152:796-802. doi:https://doi.org/10.1177/0194599815576905
- Piazza C, Grammatica A, Paderno A. Microvascular head and neck reconstruction in the elderly: the University of Brescia experience. Head Neck. 2016;38:E1488-E1492. doi:https://doi.org/10.1002/hed.24264
- Brady J, Desai S, Crippen M. Association of anesthesia duration with complications after microvascular reconstruction of the head and neck. JAMA Facial Plast Surg. 2018;20:188-195. doi:https://doi.org/10.1001/jamafacial.2017.1607
- Singh B, Cordeiro P, Santamaria E. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg. 1999;103:403-411. doi:https://doi.org/10.1097/00006534-199902000-00007
- Lin H, Huang Y, Chu Y. Vascular anatomy is a determining factor of successful submental flap raising: a retrospective study of 70 clinical cases. Peer J. 2017;5. doi:https://doi.org/10.7717/peerj.3606
- Alander J, Kaartinen I, Laakso A. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012. doi:https://doi.org/10.1155/2012/940585
- Chow T, Chan T, Chow T. Reconstruction with submental flap for aggressive orofacial cancer. Plast Reconstr Surg. 2007;120:431-436. doi:https://doi.org/10.1097/01.prs.0000267343.10982.dc
- Amin A, Sakkary M, Khalil A. The submental flap for oral cavity reconstruction: extended indications and technical refinements. Head Neck Oncol. 2011;3.
- Jiang B, Gu Y, Chen W. Submental island flaps for reconstruction of hypopharyngeal non-circumferential defects after hypopharyngeal carcinoma removal. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:1183-1185.
- Yamauchi M, Yotsuyanagi T, Ezoe K. Reverse facial artery flap from the submental region. J Plast Reconstr Aesthet Surg. 2010;63:583-588. doi:https://doi.org/10.1016/j.bjps.2009.01.035
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 999 times
- PDF downloaded - 181 times



