Head and neck
Vol. 44: Issue 5 - October 2024
Role of PET/CT in improving the cost effectiveness of nimotuzumab in nasopharyngeal carcinoma
Abstract
Objective. This study aims to use the maximum standardised uptake value (SUVmax) of 18F-fluorodeoxyglucose positron emission tomography to improve the cost effectiveness of nimotuzumab (NTZ) in locoregionally advanced nasopharyngeal carcinoma (LA-NPC).
Methods. Two hundred and forty-eigh patients with LA-NPC, who met the inclusion criteria, were recruited from January 2012 to June 2019. The survival differences and independent factors were assessed using the Kaplan-Meier method and by Cox proportional hazards regression analysis. A cost effectiveness analysis was performed.
Results. The optimal cut-off value for SUVmax was 12.92. Multivariable analysis indicated a prognostic significance of overall survival (OS) for the NTZ treatment (p = 0.023) and SUVmax (p = 0.014). The exploratory subgroup survival analysis revealed that LA-NPC patients with SUVmax > 12.92 treated with concurrent chemoradiotherapy (CCRT) and NTZ had a significantly improved 3-year OS compared to patients treated with CCRT alone (96.2 vs 73.2%, p = 0.047). Furthermore, the treatment cost for NTZ was $6,317.61. This incurred an additional cost of $274.68 for every 1% increase in OS. Conclusions. For patients with LA-NPC with SUVmax > 12.92, the addition of NTZ to CCRT can improve OS and is cost effective.
Introduction
Nasopharyngeal carcinoma (NPC) incidence, and the morbidity and mortality that arise from it in Southern China, Southeastern Asia, and North Africa, are the highest in the world with poorly differentiated or undifferentiated squamous cell carcinoma being the main histotypes 1. NPC is a highly invasive tumour, and the majority of cases (up to 70%) are locally advanced at the time of detection 2. The standard of care for locoregionally advanced nasopharyngeal carcinoma (LA-NPC) are induction chemotherapy (IC) followed by concurrent chemoradiotherapy (CCRT), CCRT followed by adjuvant chemotherapy, or CCRT alone 3,4. Although intensity-modulated radiation therapy (IMRT) has significantly improved the overall survival (OS) of locoregionally advanced cases, approximately 20-30% of patients continue to have an unfavourable prognosis due to distant metastasis, with or without recurrent disease 5. Therefore, novel treatment alternatives that can prolong the survival of LA-NPC patients are urgently warranted.
The overexpression of epidermal growth factor receptor (EGFR), which is an Erb-B receptor tyrosine kinase, occurs in nearly 70% of NPC cases 6. This may lead to a poor prognosis in NPC patients 7,8, and a high rate of treatment resistance due to tumour cell proliferation. Nimotuzumab (NTZ) is the improved version of the anti-EGFR monoclonal antibody, and this has shown promise for LA-NPC patients treated with CCRT. A large cohort study revealed that CCRT combined with NTZ can lead to better 5-year OS with tolerable side effects compared to CCRT alone (76.1 vs 72.3%, p = 0.004) in NPC patients with EGFR expression. Furthermore, NTZ is approved for the treatment of stage III-IVa NPC in combination with radiotherapy (RT) and CCRT 9. Although the addition of NTZ has exhibited significant survival benefits, TNM staging alone in LA-NPC patients who received CCRT combined with NTZ remains inadequate to determine the clinical outcomes. Thus, additional indicators are needed to guide clinical practice. Furthermore, the cost of NTZ remains an enormous financial burden, making this unlikely to be considered as cost effective for patients in China 10. Therefore, it is imperative to identify factors that lead to clear survival benefits from NTZ.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is used to stage, assess the curative effect, and predict the prognosis of patients with solid tumours 11,12. Modern imaging techniques, in addition to obvious information on the dimensions of the tumour, can provide useful metrics for biomarkers, especially when using 18F-FDG PET/CT. These metrics include metabolic tumour volume (MTV), total lesion glycolysis (TLG), and maximum standardised uptake value (SUVmax). A previous study conducted by the investigators revealed that SUVmax plays a crucial role in reflecting the invasive and metastatic potential of NPC 13. In addition, some studies have revealed that SUVmax is useful in determining the prognosis of NPC 14,15. However, no study has been conducted to determine whether SUVmax can be used to predict the treatment response of NPC patients who received NTZ. Therefore, the present retrospective study attempted to determine whether a pattern or association exists between EGFR expression of LA-NPC patients who underwent CCRT, with or without NTZ. Furthermore, the possible predictive value of SUVmax in 18F-FDG PET/CT was determined to identify high-risk patients, and further increase the cost effectiveness of NTZ in LA-NPC.
Materials and methods
Patient selection
The present retrospective study reviewed the medical records of newly diagnosed NPC patients in our centre from January 2012 to June 2019. A total of 248 patients, who satisfied the following inclusion criteria, was recruited for the present study: (1) Karnofsky performance score of ≥ 90; (2) histologically confirmed NPC; (3) immunohistochemically confirmed EGFR expression; (4) stage III-IVb NPC, as described by the American Joint Committee on Cancer 7th TNM staging system; (5) IC followed by CCRT, with or without NTZ; (6) available 18F-FDG PET/CT data prior to IC; (7) no history of malignant disease; (8) no targeted therapy with any other anti-EGFR antibody.
Radiotherapy and chemotherapy
All patients received IMRT. The target volume, at-risk organ(s), and dose for the RT were delineated using a previously described treatment protocol 16. A total radiation dose of 69.7-70 Gy given over 33-35 fractions was prescribed for the primary gross tumour planning target volume (PTV) or the gross tumour volume, when lymph nodes were involved. The high-risk region (CTV1) had a recommended dose of 62-62.7 Gy, while the low-risk region (CTV2) was given 54.4-56.2 Gy, in corresponding fractions. A margin of 3 mm was added to determine the PTV. Then, 2-3 cycles of IC were administered to all patients prior to CCRT. The IC regimen used was TP (paclitaxel 135 mg/m2 on day 1 + cisplatin 80 mg/m2 on day 2) and GP (gemcitabine 1,000 mg/m2 on days 1 and 8 + cisplatin 80 mg/m2 on day 2). Cisplatin (80 mg/m2) was used for the CCRT regimen. The IC and CCRT regimens were administered through the intravenous route and were repeated every three weeks. RT was simultaneously administered at the first cycle of CCRT.
Nimotuzumab
The full dose of NTZ was administered to 110 patients, according to the decision of the patient to receive the medication, i.e. 200 mg of NTZ was intravenously administered, and this was repeated once a week for seven weeks prior to IMRT.
18-FDG PET/CT imaging
All 248 NPC patients in the present study underwent 18F-FDG PET/CT using a Gemini TF 64 PET/CT scanner (Philips, Holland). These patients were placed in the supine position and were instructed to fast for at least six hours prior to the 18F-FDG PET/CT, in order to maintain a blood glucose level within 3.9-6.5 mmol/L. A dose of 148-296 MBq of 18F-FDG was intravenously administered. The scan was performed from the head to the proximal thigh, using the following parameters: slice width 4 mm, 140 kVp, 2.5 mA, and matrix 512×512. The iterative reconstruction of the 18F-FDG PET/CT image was performed by CT-based attenuation correction. The region of interest of the tumour lesion was used to define the 18F-FDG SUVmax, and this was calculated as the ratio of decay-corrected tissue activity (nCi/mL) to the sum of the injected dose of FDG (nCi) and body weight (g) of the patient. The highest SUV of the primary tumour was calculated as the SUVmax.
Clinical endpoints and follow-up
Regular reviews were carried out at intervals of three months in the first two years, followed by intervals of six months during the next five years, and subsequently once a year. The routine surveillance included the following: physical examination, haematological and biochemical tests, nasopharyngoscopy, enhanced magnetic resonance imaging of the head and neck, CT of the thorax, and ultrasound (USG) of the abdomen. The clinical endpoints were documented up to January 2022.
OS (defined as the time from day one after diagnosis to death due to any cause) and progression-free survival (PFS, defined as the time from day one after diagnosis to disease progression or death due to any cause) were the primary clinical endpoints. The other endpoints were: local recurrence-free survival (LRFS, defined as the time from day one after diagnosis to local recurrence), regional recurrence-free survival (RRFS, defined as the time from day one after diagnosis to regional recurrence), locoregional relapse-free survival (LRRFS, defined as the time from day one after diagnosis to local or regional recurrence or both), and distant metastasis-free survival (DMFS, defined as the time from day one after diagnosis to the first distant metastasis). The period between the date of diagnosis and each event or the last review was defined as the clinical endpoint.
Statistical analysis
SPSS (version 23.0; IBM Corp, Armonk, NY, USA), GraphPad Prism (version 9.3.0, GraphPad Software, San Diego, California, USA), and R software (version 4.0.5, The R Foundation for Statistical Computing) were used to analyse the recorded data. The receiver operating characteristic (ROC) curve was used to measure predictive value of SUVmax in predicting NPC patients’ survival outcomes, and the maximal Jordan index (sensitivity + specificity – 1) was used to calculate the SUVmax value to determine the optimal value for predicting survival. SUVmax was converted into classification variables using ROC curve. Age was converted into classification variables using a common clinical parameter 17. Differences between the CCRT alone group and CCRT with NTZ group were compared using Chi-square test. The maximal Jordan index was used to determine the optimal cut-off value for SUVmax in prognosis. The Kaplan-Meier method was used to determine OS, PFS, LRFS, RRFS, LRRFS, and DMFS. The differences in OS, PFS, LRFS, RRFS, LRRFS, and DMFS between groups were calculated using log-rank test. The variables that achieved significance in univariable analysis including SUVmax and the use of NTZ and the variables based on the previous study including age, gender, and TNM stage were further investigated in the multivariable analysis. Multivariable analysis was performed using Cox proportional hazards regression analysis. The hazard ratio and 95% confidence interval (CI) were estimated for each factor. All tests were two-sided, and a p-value of < 0.05 was considered statistically significant.
Using the exchange rate as of January 2022 (1 USD = 6.36 Chinese Yuan [CNY]), all cost calculations were performed in US dollars (USD). The Diagnosis Related Groups (DRG) issued by the Medical Insurance Administration Bureau of China was used to determine the total cost of the CCRT. The total cost of the CCRT was $10,849.06, which included medical service fees, anti-cancer drugs, RT, treatments for chemoradiotherapy-related adverse events, and radiographic examination or routine blood tests. The price for NTZ was $902.52 per week. The total cost of NTZ was not included in the total cost of the CCRT.
The cost effectiveness ratio (C/E%) and the incremental cost effectiveness ratio (ICER) were used to determine the outcome measures for the cost effectiveness analysis. The C/E% was calculated by dividing the total treatment cost by the effectiveness. ICER was defined as the total cost difference per patient between the CCRT with NTZ group and CCRT alone group, divided by the difference in effectiveness between the two groups. China’s per capita gross domestic product (GDP) was $13,474.84 in the year 2022. Willingness-to-pay (WTP) is the threshold performed in economic predictions which helps calculate the greatest amount for every additional unit of effectiveness gained form a new intervention 18. An increase of 1% in OS rate with the cost of the GDP in 2022 would mean an additional cost of $134.75. Cost effectiveness acceptability analysis was conducted to evaluate optimal strategies at given WTP thresholds. The WTP threshold was set as three times the GDP per capita with an increase of 1% in OS rate, which was $404.25 in 2022 in China 13.
Results
Patient demographics
The clinical data of 5,511 consecutive newly diagnosed NPC cases were reviewed. In all, 248 LA-NPC patients (177 males and 71 females) who satisfied inclusion criteria were recruited for the present study (Fig. 1). The basic characteristics of all patients are summarised in Table I. The median follow-up time was 42 months (range: 6-96 months). The median age of these patients was 47 years, with an age range of 19-83 years. The absolute numbers and percentages of patients according to the extent of the primary tumour (T1, T2, T3, or T4) were 35 (14.1%), 36 (14.5%), 124 (50%), and 53 (21.4%), respectively. The regional lymph node distribution for N0, N1, N2, and N3 was 14 (5.6%), 71 (28.6%), 93 (37.5%), and 70 (28.3%), respectively. For patients < 65 and ≥ 65 years old, the distribution was 228 (91.9%) and 20 (8.1%), respectively. The distribution for deaths, regional recurrence, local recurrence, local-regional relapse, and distant metastasis was 19 (7.6%), 13 (5.2%), 27 (10.9%), 35 (14.1%), and 30 (12.1%), respectively.
Impact of nimotuzumab on overall survival
Among the 55 LA-NPC patients with SUVmax >12.92, 29 who were treated with chemoradiotherapy alone were assigned to the CCRT alone group, while 26 who received chemoradiotherapy and NTZ were assigned to the CCRT with NTZ group. Among the 193 LA-NPC patients with SUVmax ≤ 12.92, 109 patients who were treated with CCRT alone were assigned to the CCRT alone group, while 84 patients who received CCRT and NTZ were assigned to the CCRT with NTZ group. The clinical characteristics of patients treated with CCRT alone and patients treated with CCRT and NTZ did not significantly differ (Tab. II). Patients who received CCRT plus NTZ had a better 3-year OS compared to patients who only received CCRT (97.2 vs 91%; p = 0.018, Figure 2A), as determined by Kaplan-Meier analysis. No significant difference was found for 3-year PFS (83.3 vs 80.1%; p = 0.407), 3-year LRFS (96.3 vs 89.2%; p = 0.193), 3-year RRFS (95.4 vs 86.4%; p = 0.086), 3-year LRRFS (98.2 vs 93%; p = 0.121), and 3-year DMFS (87 vs 90.1%; p = 0.517) of patients who received NTZ, respectively.
Impact of SUVmax on overall survival
The average SUVmax for the entire cohort was 10.33 ± 5.69 (range: 1.70-48.88). The area under concentration-time curve for SUVmax was 0.596, and the sensitivity and specificity were 0.438 and 0.920, respectively, based on the ROC curve analysis. The optimal cut-off value was 12.92, based on the Jordan index. Kaplan-Meier analysis revealed that SUVmax ≤ 12.92 had superior 3-year OS (95.7 vs 87.2%; p = 0.29, Figure 2B), 3-year PFS (84.7 vs 59.7%; p = 0.034, Figure 2C), and 3-year LRRFS (97.3 vs 87.5%; p = 0.039, Figure 2D) compared to SUVmax > 12.92, respectively. There was no significant difference in 3-year LRFS (95.2 vs 85.3%; p = 0.127), 3-year RRFS (93.1 vs 75.4%; p = 0.062), and 3-year DMFS (90.9 vs 79.3%; p = 0.121), according to the SUVmax ≤ 12.92 compared with SUVmax > 12.92. Prognosis was thus significantly better for SUVmax ≤ 12.92 when NTZ was used in LA-NPC considering OS, PFS, and LRRFS, which could affect the indices like LRFS, RRFS and DMFS, although this was not observed in statistical analysis.
Multivariable analysis
Cox regression models were used to analyse the OS and PFS of the 248 patients in the present study. Using univariable analysis and previous study results 10, age, gender, TNM stage, SUVmax, and use of NTZ were further investigated in multivariable analysis. The results demonstrated that SUVmax ≤ 12.92 (p = 0.014) and treatment with NTZ (p = 0.023) were independent prognostic factors for better OS. Furthermore, SUVmax ≤ 12.92 was an independent prognostic factor for better PFS (p = 0.045) (Tab. III, Figs. 3A-B).
Survival analysis between nimotuzumab and SUVmax in subgroups
According to multivariate analysis, NTZ and SUVmax were independent prognostic factors for OS. An exploratory subgroup survival analysis was conducted after stratification by SUVmax (≤ 12.92 and > 12.92) to identify the stage III-IVb NPC patients who may benefit from NTZ, and determine whether the NTZ treatment was optimal for NPC patients with SUVmax > 12.92. Patients with SUVmax > 12.92 who received CCRT and NTZ had superior 3-year OS compared to patients with SUVmax > 12.92 who received CCRT alone (96.2 vs 73.2%; p = 0.047, Figure 4A). No significant difference was found in 3-year PFS for patients with SUVmax > 12.92 who received CCRT and NTZ compared to those who received CCRT only (60 vs 57.1%; p = 0.433). For patients with SUVmax ≤ 12.92 who received CCRT and NTZ, no significant difference was found for 3-year OS (97.6 vs 94.3, p = 0.129, Figure 4B) or 3-year PFS (84.1 vs 80%; p = 0.563) compared to patients with SUVmax ≤ 12.92 who received CCRT only. For stage III-IVb NPC patients with SUVmax > 12.92, the 3-year OS increased by 23% in the ‘CCRT with NTZ’ group compared to the ‘CCRT alone’ group. This demonstrates that compared to the corresponding subgroups, NTZ treatment benefited patients with SUVmax > 12.92. For patients with SUVmax ≤ 12.92 who were treated with NTZ, there was no significant difference in OS (Tab. IV).
Cost effectiveness and sensitivity analysis
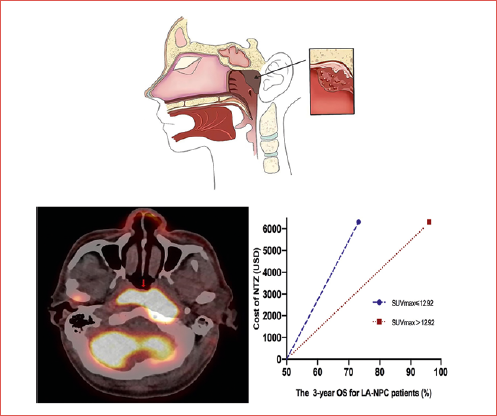
For stage III-IVb NPC patients, the total treatment cost for CCRT and NTZ per patient was $17,166.67, while the total treatment cost for only CCRT per patient was $10,849.06. The extra treatment cost for NTZ was $6,317.61. The C/E% for the 3-year OS of patients in the CCRT with NTZ group with SUVmax > 12.92 vs the CCRT alone group with SUVmax > 12.92 was $178.45 and $148.21, respectively. The difference in effectiveness for 3-year OS with SUVmax > 12.92 was 23%. Therefore, the ICER was only $274.68. This translates to an increase of 1% in OS rate for NPC patients with SUVmax > 12.92 after costing an additional $274.68 (less than the WTP threshold of $404.25) (Fig. 5). However, in the entire cohort, the ICER was $1,018.96 (more than the WTP threshold of $404.25).
In the sensitivity analysis, OS varied by ± 10%, yielding a C/E% for the 3-year OS of patients in the CCRT with NTZ group vs. the CCRT alone group of $199.15 vs $130.40, respectively.
Discussion
The current study retrospectively analysed the treatment efficacy in 248 patients with stage III-IVb EGFR positive NPC who received standard CCRT with or without NTZ. Similar to previous studies, the addition of NTZ to standard CCRT conferred significant survival benefit for LA-NPC patients. The 3-year OS in the CCRT plus NTZ group was significantly greater than patients who only received CCRT (97.2 vs 91%; p = 0.018). The optimal cut-off value of SUVmax in predicting the survival outcome in NPC patients was 12.92. In multivariable analysis, SUVmax and the addition of NTZ were significant prognostic factors for OS. Moreover, patients with SUVmax > 12.92 who received targeted therapy with the addition of NTZ presented with a clear survival benefit (96.2 vs 73.2%; p = 0.047). The previous study conducted by the investigators revealed that the additional cost for every 1% increase in the OS rate was $2,052.09. Thus, this could not be considered as cost-effective 10. The high-risk factor for LA-NPC in the present study was SUVmax > 12.92. Thus, NTZ may help to improve the outcomes of high-risk LA-NPC patients. The difference in cost-effectiveness for the 3-year OS of stage III-IVa NPC patients with SUVmax > 12.92 was 23% higher. The extra treatment cost of NTZ was $6,317.61. Therefore, the ICER was calculated as $274.68. This means that the additional cost of treatment was only $274.68 for every 1% improvement in OS rate. The WTP threshold was set as three times the GDP per capita with an increase of 1% in OS rate in 2022, which was $404.25 in China. The additional cost of NTZ in the study was significantly lower than WTP. Thus, NTZ can be cost-effective and may be recommended for patients with LA-NPC and SUVmax > 12.92.
NPC arises from the epithelial cells in the nasopharynx and is a very aggressive malignancy. In 2020, the International Agency for Research on Cancer reported 133,354 new cases and 80,008 new cancer-related deaths due to NPC worldwide. At present, the National Comprehensive Cancer Network (NCCN) guidelines recommend IC with CCRT, CCRT, or CCRT plus adjuvant chemotherapy as the standard treatment for stage III-IVb (locoregionally advanced) NPC. Even with precision RT and standard cisplatin-based chemotherapy, prognosis of patients with LA-NPC remains unsatisfactory, and 20-30% have recurrent disease and/or distant metastasis. Due to the significant improvement in pathophysiology of the disease, a number of tumour gene targets have drawn the attention of researchers. Targeted therapy with an EGFR antibody has shown promise in improving the prognosis of patients with NPC. EGFR is an Erb-B receptor tyrosine kinase, and is widely overexpressed in head and neck cancer. Its intracellular signal transduction leads to enhanced cell proliferation, inhibition of apoptosis, increased angiogenesis, and promotion of metastasis. A recent study reported that high EGFR expression can lead to early recurrence and low OS, combined with a low disease-free survival (DFS), in stage II-IV head and neck tumours 19. Anti-EGFR monoclonal antibodies, including cetuximab (CTX) and NTZ, can specifically inhibit EGFR, and enhance radio-sensitivity, increasing the effect of synchronous chemoradiotherapy in NPC patients 20. A large retrospective cohort study with a long follow-up period revealed that CTX/NTZ treatment combined with CCRT can significantly prolong the OS of patients with stage II-IVb NPC compared to CCRT alone (3-year OS: 96.6 vs 92.9%, p = 0.015) and DFS (3-year DFS: 93.5 vs 89.3%, p = 0.03). However, significant differences in acute toxicities, such as skin reaction and mucositis, were observed in patients who received CTX and CCRT compared to NTZ and CCRT, or CCRT alone 9. NTZ is an improved and humanised anti-EGFR monoclonal antibody with a preferential tumour cell uptake compared to normal tissue. Furthermore, this has significantly fewer adverse effects on normal mucosa and skin. In the past five years, a number of clinical studies have revealed better clinical outcomes and less radiation-related toxicity for CCRT in combination with NTZ in patients with LA-NPC 21,22. This is similar to the present study, in which patients who received CCRT plus NTZ had a superior OS compared to patients who received CCRT alone (3-year OS: 97.2 vs 91%), and the difference was statistically significant. However, the TNM stage of LA-NPC patients remained the same, and for those who received CCRT in combination with NTZ clinical outcomes greatly varied. This shows that by itself TNM stage is not adequate in the application of NTZ, without considering other clinical factors. Thus, it is necessary to explore other important prognostic indicators in order to identify which stage III-IV NPC patents can achieve a survival benefit from NTZ.
The relationship between the image-derived biomarkers in 18F-FDG PET (SUVmax, TLG, and MTV) and clinical outcomes in solid tumours has been extensively studied. Feng et al. reported on 50 patients with oesophageal carcinoma in which post-neoadjuvant CCRT SUVmax ≥ 3 was a poor prognostic factor for DFS (HR = 3.417, p = 0.011) and OS (HR = 3.665, p = 0.013) 23. In another retrospective study, Di Stasio et al. reported that SUVmax ≥ 10.08, MTV ≥ 27.89, and TLG ≥ 134.85 conferred a significantly worse prognosis in 49 patients with pleomorphic lung carcinoma, and that the metabolic biomarkers mentioned above can be used to predict recurrence and death 24. Burchardt et al. conducted a multivariate Cox regression analysis on 93 patients with locally advanced squamous cervical cancer who received radical cisplatin-based chemoradiotherapy, and found that SUVmax > 12.6 and TLG > 245.7 were independent predictors for OS 25. Lin et al. investigated 108 NPC patients who received chemoradiotherapy, and found that pre-treatment SUV > 8.35 can lead to a significantly worse 3-year OS compared to a lower SUV 26. The pre-treatment SUV can be used to independently predict adverse 3-year DFS and OS, which is similar to a previous study conducted by the same investigators, and the best cut-off value for SUVmax was 8.2, as derived from the ROC curve. Furthermore, NPC patients with SUVmax < 8.2 appeared to have a clinical benefit (3-year PFS, 91.1 vs 73%, p = 0.027) 26.
In addition, other studies have focused on the relationship between image-derived biomarkers using 18F-FDG PET/CT for solid tumours and the response to chemoradiotherapy. Dae et al. reported that after neoadjuvant chemoradiotherapy (nCRT), the SUVmax, TLG, and MTV may be used to predict pathological complete response (pCR) 23. Furthermore, that study assessed the SUVmax, TLG, and MTV, before and after nCRT, in 137 patients with locally advanced rectal cancer, and it was found that the post-SUVmax, post-TLG, and post-MTV were smaller in the pCR group compared to the non-pCR group. Multivariate analysis revealed that post-SUVmax, post-TLG, and post-MTV were independent factors for pCR 27. Asif et al. reported that the percentage decrease in SUVmax after nCRT can be used as a predictor for pCR in locally advanced oesophageal squamous cell carcinoma patients who received nCRT 28. No study has reported the predictive value(s) when using image-derived biomarkers in 18F-FDG PET/CT to assess the response to treatment of NPC patients who received NTZ. The SUVmax has the advantage of repeatability, objectivity, and steady parameters. Thus, the present study evaluated the predictive value of SUVmax in selecting which stage III-IVb NPC patients might benefit from the addition of NTZ to the treatment protocol. The results revealed that stage III-IVb NPC patients with SUVmax > 12.92 had the worse 3-year OS compared to patients with SUVmax ≤ 12.92 (87.2 vs 95.7%, p = 0.029). The further subgroup analysis revealed that for stage III-IVb NPC patients with SUVmax > 12.92, the CCRT plus NTZ treatment resulted in a higher 3-year OS compared to CCRT alone. However, for stage III-IVb NPC patients with SUVmax ≤ 12.92, there was no significant difference in 3-year OS between the two groups. Thus, NTZ treatment benefited stage III-IVb patients with SUVmax > 12.92.
To the best of our knowledge, the present study is the first to assess the predictive value of SUVmax to justify the addition of NTZ to CCRT in patients with LA-NPC and identify high-risk patients who would benefit from NTZ. Furthermore, the cost effectiveness of the addition of NTZ in high-risk stage III-IVb EGFR-positive NPC patients was demonstrated in our study, although the validity of this assertion needs to be confirmed with larger cohorts, as our study has some limitations. First, the number of stage III-IVb NPC cases included was limited to a single institution, and more data will be required to verify our results. Second, due to the small number of cases included in the study, larger studies are needed to provide more definitive evidence. Third, selection bias inherent in retrospective studies may have reduced the overall validity of our results. The limitations of the present single institution study can be resolved through a larger multicentre, longitudinal, and randomised controlled design.
Conclusions
In summary, OS of LA-NPC patients with SUVmax >12.92 significantly improved, and was cost-effective, when NTZ was added to CCRT. However, the addition of NTZ to CCRT was not cost-effective in LA-NPC patients with SUVmax ≤ 12.92. The addition of NTZ therefore seems to confer a clear survival benefit in a selected group of patients, and needs to be confirmed with larger, multicentre and randomised clinical trials.
Acknowledgments
The authors would like to thank all patients who participated in the study.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
The study was supported in part by grants from the Natural Science Foundation of Fujian Province (Grant/Award number: 2020J011124), and the Bethune-Translational Medicine Research Fund for Oncology Radiotherapy (Grant/Award number: flzh202126).
Author contributions
All authors listed have made substantial, direct, and intellectual contributions to the work, and approved the manuscript for publication.
Ethical consideration
The retrospective study was approved by the Fujian Province Cancer Hospital Institutional Review Board (No. YKT2020-011-01), and was conducted strictly following the Declaration of Helsinki of the World Association guidelines. All patients provided written informed consents prior to treatment, and all information was anonymized prior to analysis.
Availability of data and materials
The data used and/or analysed in the study are available from the corresponding author.
History
Received: September 22, 2023
Accepted: May 13, 2024
Figures and tables
Figura 1. Study flow diagram.
Figure 2. Survival curves for the 248 patients with LA-NPC, according to the following: (a) OS in the CCRT group vs CCRT+NTZ group, (b) OS for SUVmax > 12.92 vs SUVmax ≤ 12.92, (c) PFS for SUVmax > 12.92 vs SUVmax ≤ 12.92, and (d) LRRFS for SUVmax > 12.92 vs SUVmax ≤ 12.92.
Figure 3. Forest plots for multivariate analysis of (A) OS and (B) PFS of the 248 patients with LA-NPC. HR, hazard ratio.
Figure 4. Survival curves for (A) the 193 LA-NPC patients with SUVmax > 12.92 in the CCRT group vs CCRT+NTZ group, and (B) the 55 LA-NPC patients with SUVmax ≤ 12.92 in the CCRT group vs CCRT+NTZ group.
Figure 5. The difference in effectiveness (3-year OS) for LA-NPC patients treated with CCRT plus NTZ, which was stratified by SUVmax ≤ 12.92 and SUVmax > 12.92.
| Parameter | Number of patients (%) |
|---|---|
| Gender | |
| Male | 177 (71.4) |
| Female | 71 (28.6) |
| Age (years) | |
| < 65 | 228 (91.9) |
| ≥ 65 | 20 (8.1) |
| Primary tumour category | |
| T1 | 35 (14.1) |
| T2 | 36 (14.5) |
| T3 | 124(50) |
| T4 | 53 (21.4) |
| Regional lymph nodes category | |
| N0 | 14 (5.6) |
| N1 | 71 (28.6) |
| N2 | 93 (37.5) |
| N3 | 70 (28.3) |
| Pattern of failure | |
| Death | 19 (7.6) |
| Local-regional relapse | 13 (5.2) |
| Local recurrence | 27 (10.9) |
| Distant metastasis | 30 (12.1) |
| Characteristic | CCRT alone | CCRT plus NTZ | X2 | p value |
|---|---|---|---|---|
| Gender | 2.426 | 0.119 | ||
| Male | 104 | 73 | ||
| Female | 34 | 37 | ||
| Age (years) | 0.771 | 0.380 | ||
| < 65 | 125 | 103 | ||
| ≥ 65 | 13 | 7 | ||
| Primary tumour category | 1.273 | 0.736 | ||
| T1 | 21 | 14 | ||
| T2 | 20 | 16 | ||
| T3 | 65 | 59 | ||
| T4 | 32 | 21 | ||
| Regional lymph nodes category | 0.468 | 0.926 | ||
| N0 | 7 | 7 | ||
| N1 | 39 | 32 | ||
| N2 | 54 | 39 | ||
| N3 | 38 | 32 | ||
| SUVmax | 0.244 | 0.621 | ||
| < 12.92 | 109 | 84 | ||
| ≥ 12.92 | 29 | 26 | ||
| LA-NPC: locoregionally advanced nasopharyngeal carcinoma; CCRT: concurrent chemoradiotherapy; NTZ: nimotuzumab; SUV max : maximum standardised uptake value. | ||||
| OS | PFS | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p value | |
| Gender | ||||||
| Male | 1 | 1 | ||||
| Female | 0.948 | 0.332-2.709 | 0.921 | 0.794 | 0.444-1.418 | 0.435 |
| Age | ||||||
| < 65 years | 1 | 1 | ||||
| ≥ 65 years | 2.000 | 0.427-9.357 | 0.379 | 0.360 | 0.087-1.491 | 0.159 |
| T category | ||||||
| T1 | 1 | 0.979 | 1 | 0.512 | ||
| T2 | 1.308 | 0.257-6.653 | 0.746 | 0.563 | 0.204-1.556 | 0.268 |
| T3 | 0.898 | 0.149-5.431 | 0.907 | 0.759 | 0.346-1.668 | 0.493 |
| T4 | 1.039 | 0.340-3.174 | 0.946 | 1.040 | 0.443-2.443 | 0.927 |
| N category | ||||||
| N0 | 1 | 0.090 | 1 | 0.117 | ||
| N1 | 0.0983 | 0.000-0.000 | 0.983 | 2.630 | 0.610-11.331 | 0.195 |
| N2 | 0.348 | 0.105-1.151 | 0.084 | 1.803 | 0.407-7.993 | 0.438 |
| N3 | 0.222 | 0.065-0.756 | 0.016 | 3.493 | 0.793-15.385 | 0.098 |
| Nimotuzumab | ||||||
| Without | 1 | 1 | ||||
| With | 0.232 | 0.066-0.861 | 0.023 | 0.823 | 0.487-1.389 | 0.465 |
| SUVmax (T) | ||||||
| < 12.92 | 1 | 1 | ||||
| ≥ 12.92 | 3.736 | 1.300-10.734 | 0.014 | 1.787 | 1.014-3.150 | 0.045 |
| OS: overall survival; PFS: progression-free survival; HR: hazard ratio; 95% CI: confidence interval; SUV max : maximum standardised uptake value. | ||||||
| SUVmax | Treatment | N (%) | 3-year OS | p-value | 3-year PFS | p value |
|---|---|---|---|---|---|---|
| ≤ 12.92 | CCRT alone | 109 (44%) | 94.3% | 0.129 | 80% | 0.563 |
| ≤ 12.92 | CCRT plus NTZ | 84 (33.9%) | 97.6% | 84.1% | ||
| > 12.92 | CCRT alone | 29 (11.7%) | 73.2% | 0.047 | 57.1% | 0.433 |
| > 12.92 | CCRT plus NTZ | 26 (10.4%) | 96.2% | 60% |
References
- Bray F, Ferlay J, Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. doi:https://doi.org/10.3322/caac.21492
- He T, Yan R, Chen H. Comparing the 7th and 8th editions of UICC/AJCC staging system for nasopharyngeal carcinoma in the IMRT era. BMC Cancer. 2021;21. doi:https://doi.org/10.1186/s12885-021-08036-8
- Cao S, Yang Q, Guo L. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14-23. doi:https://doi.org/10.1016/j.ejca.2016.12.039
- Dong Y, Xiang C, Lu J. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma. Strahlentherapie Und Onkologie. 2016;192:1-9. doi:https://doi.org/10.1007/s00066-016-0970-3
- Ribassin-Majed L, Marguet S, Lee A. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017;35:498-505. doi:https://doi.org/10.1200/jco.2016.67.4119
- Sun Y, Wang Y, Guan L. A systematic analysis in efficacy and safety of nimotuzumab combined with chemoradiotherapy in treatment of advanced nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2023;280:1183-1190. doi:https://doi.org/10.1007/s00405-022-07609-y
- Chen X, Liang R, Zhu X. Anti-EGFR therapies in nasopharyngeal carcinoma. Biomed Pharmacother. 2020;131. doi:https://doi.org/10.1016/j.biopha.2020.110649
- Manoli A, Katsinis S, Roukas D. EGFR mutational landscape in nasopharyngeal carcinoma. J BUON. 2021;26.
- Cai Z, Chen D, Qiu W. Concurrent chemoradiotherapy combined with nimotuzumab in stage III-IVa nasopharyngeal carcinoma: a retrospective analysis. J Cancer Res Clin Oncol. 2023;149:2327-2344. doi:https://doi.org/10.1007/s00432-022-04355-w
- Fei Z, Xu T, Li M. Effectiveness and cost-effectiveness analysis of nimotuzumab for the radiotherapy of locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol. 2020;15. doi:https://doi.org/10.1186/s13014-020-01674-5
- Sun G, Cheng C, Li X. Metabolic tumor burden on postsurgical PET/CT predicts survival of patients with gastric cancer. Cancer Imaging. 2019;19. doi:https://doi.org/10.1186/s40644-019-0205-9
- Nie P, Yang G, Wang N. Additional value of metabolic parameters to PET/CT-based radiomics nomogram in predicting lymphovascular invasion and outcome in lung adenocarcinoma. Eur J Nucl Med Mol Imaging. 2021;48:217-230. doi:https://doi.org/10.1007/s00259-020-04747-5
- Chen C, Xu T, Qiu X. Selectively recommend 18F-FDG PET/CT for patients with de novo nasopharyngeal carcinoma in endemic areas. Radiat Oncol. 2021;16. doi:https://doi.org/10.1186/s13014-021-01954-8
- Lin H, Chan S, Cheng N. Pretreatment 18F-FDG PET/CT texture parameters provide complementary information to Epstein-Barr virus DNA titers in patients with metastatic nasopharyngeal carcinoma. Oral Oncol. 2020;104. doi:https://doi.org/10.1016/j.oraloncology.2020.104628
- Gu B, Zhang J, Ma G. Establishment and validation of a nomogram with intratumoral heterogeneity derived from 18F-FDG PET/CT for predicting individual conditional risk of 5-year recurrence before initial treatment of nasopharyngeal carcinoma. BMC Cancer. 2020;20. doi:https://doi.org/10.1186/s12885-020-6520-5
- Chen C, Fei Z, Pan J. Significance of primary tumor volume and T-stage on prognosis in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Jpn J Clin Oncol. 2011;41:537-542. doi:https://doi.org/10.1093/jjco/hyq242
- Liu H, Chen Q, Guo L. Feasibility and efficacy of chemoradiotherapy for elderly patients with locoregionally advanced nasopharyngeal carcinoma: results from a matched cohort analysis. Radiat Oncol. 2013;8. doi:https://doi.org/10.1186/1748-717X-8-70
- Shao T, Ren Y, Zhao M. Cost-effectiveness analysis of camrelizumab plus chemotherapy as first-line treatment for advanced squamous NSCLC in China. Front Public Health. 2022;10. doi:https://doi.org/10.3389/fpubh.2022.912921
- Ramos T, Fernández B, Herrera Z. Nimotuzumab for patients with inoperable cancer of the head and neck. Front Oncol. 2020;10. doi:https://doi.org/10.3389/fonc.2020.00817
- Mao L, Tan J, Wang F. Retrospective study comparing anti-EGFR monoclonal antibody plus cisplatin-based chemoradiotherapy versus chemoradiotherapy alone for stage II-IVb nasopharyngeal carcinoma and prognostic value of EGFR and VEGF expression. Clin Otolaryngol. 2019;44:572-580. doi:https://doi.org/10.1111/coa.13340
- Zhi-Qiang W, Qi M, Ji-Bin L. The long-term survival of patients with III-IVb stage nasopharyngeal carcinoma treated with IMRT with or without Nimotuzumab: a propensity score-matched analysis. BMC Cancer. 2019;19. doi:https://doi.org/10.1186/s12885-019-6156-5
- Fangzheng W, Chuner J, Zhiming Y. Long-term use of nimotuzumab in combination with intensity-modulated radiotherapy and chemotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma: experience of a single institution. Oncol Res. 2017;26:277-287. doi:https://doi.org/10.3727/096504017X15079846743590
- Feng W, Chen Y, Kuo Y. Prognostic factors associated with 18FDG-PET/CT in esophageal squamous cell carcinoma after trimodality treatment. BMC Cancer. 2022;22:1-8. doi:https://doi.org/10.1186/s12885-022-09852-2
- Di Stasio G, Travascio L, Colandrea M. Prognostic value of PET parameters in patients with pleomorphic lung cancer: results from a single institution. Lung Cancer. 2021;158:40-46. doi:https://doi.org/10.1016/j.lungcan.2021.05.027
- Burchardt E, Burchardt W, Kubiak A. Pretreatment [18F]FDG PET/CT prognostic factors in patients with squamous cell cervical carcinoma FIGO IIIC1. Diagnostics (Basel). 2021;11. doi:https://doi.org/10.3390/diagnostics11040714
- Lin C, Hsieh T-C, Chen T-T. Predictive values of (18)f-FDG PET standardized uptake value for adjuvant chemotherapy in patients with nasopharyngeal carcinoma. J Clin Oncol. 2013;31. doi:https://doi.org/10.1200/jco.2013.31.15_suppl.6052
- Pyo D, Chio J, Lee W. A nomogram for predicting pathological complete response to neoadjuvant chemoradiotherapy using semiquantitative parameters derived from sequential PET/CT in locally advanced rectal cancer. Front Oncol. Published online 2021. doi:https://doi.org/10.3389/fonc.2021.742728
- Iqbal S, Goel S, Aggarwal A. Use of (18)F fluorodeoxyglucose positron emission tomography computed tomography in assessing response to neoadjuvant chemoradiation and its impact on survival in esophageal squamous cell carcinoma. J Gastrointest Cancer. 2021;52:1073-1080. doi:https://doi.org/10.1007/s12029-020-00543-4
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2024 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1092 times
- PDF downloaded - 253 times



