Laryngology
Vol. 44: Issue 6 - December 2024
Maximising efficiency with exoscopic surgery: a versatile approach for transoral laryngeal and oropharyngeal procedures
Abstract
Objective. Several devices have been developed to improve head and neck surgery. 3D exoscopes provide surgeons a viable alternative to microscopes. We propose our setting for transoral exoscopic oropharyngeal (TOEOS) and transoral exoscopic laryngeal surgery (TOELS).
Methods. A case series of patients treated with the exoscopic setup at the Otolaryngology Unit of IRCCS San Martino Hospital, Genoa, is presented. Our surgical setup and surgical and oncological outcomes are described.
Results. Among 40 patients undergoing TOELS for glottic and supraglottic tumours, negative superficial and deep margins were achieved in 79.2% and 75% of patients, respectively. The mean operative time was 73.7 ± 35.9 minutes. Fourteen patients were treated by TOEOS and in only one case was re-resection required due to a positive deep margin. The mean operative time for TOEOS was 140.3 ± 82.1 minutes and the average duration of hospitalisation was 10.3 ± 3.8 days.
Conclusions. 3D exoscopes improve visualisation of the surgical site in different environments and allow the use of multiple surgical instruments and lasers, easing transoral surgery. In addition, as the first surgeon’s view is shared between the operatory room (OR) staff, the exoscopic setup plays a crucial role in the collaboration between the OR team and for teaching purposes.
Introduction
Numerous devices have been developed to enhance the quality of surgical field visualisation in head and neck (H&N) surgery 1. These innovations include the employment of robotic devices in transoral (TORS) and transcervical surgery 2 as well as exoscopes. These latter consist of a high-definition (HD) camera with optical and digital zoom coupled with a light source, which allow a 3D vision 3 and the possibility to connect with robotic arms and controls 4. Parallel to this, the development of customised supports for CO2 laser micromanipulator allows performing exoscopic-assisted transoral laser surgery in alternative to use of conventional optical microscopes (OM) 5. The latter still provides excellent illumination and magnification but allows a 3D surgical field visualisation to the operating surgeon only. In contrast, the 3D exoscope displays images on a large monitor, allowing detailed visualisation of the surgical field and stereoscopic images while wearing 3D glasses to the entire operating room (OR) staff 6.
This technology was first applied in neurosurgery, urology, and gynaecology 6, and was recently introduced in laryngology 4,7,8, H&N reconstruction, ear surgery 9 and skull base surgery 10.
In the present study, we examined the application of a 3D exoscope coupled with a robotic cruise system in various surgical settings as an alternative to the traditional OM. In particular, the aim of the present paper is to describe our surgical setting with this type of technology and report the advantages and limits of the 3D exoscope in transoral laryngeal and oropharyngeal surgery.
Materials and methods
This is a case series of patients treated at the Unit of Otolaryngology of IRCCS San Martino Hospital, Genoa, between February 2019 and January 2023. We analysed two groups of patients who underwent different exoscope-assisted treatments: transoral exoscopic laryngeal surgery (TOELS) and transoral exoscopic oropharyngeal surgery (TOEOS).
All oncological patients had been submitted to surgery after multidisciplinary team discussion between surgeons, radiation and medical oncologists. The diagnostic work-up included an in-office transnasal endoscopy and an intraoperative rigid endoscopy by 0° and 70° telescopes with white light and narrow band imaging (Olympus Medical System Corporation, Tokyo, Japan) 11. Preoperative imaging included computed tomography or magnetic resonance to classify tumours according to the 8th Edition of the AJCC UICC TNM staging system 12. Surgical outcomes are described; continuous variables are reported as mean value ± standard deviation.
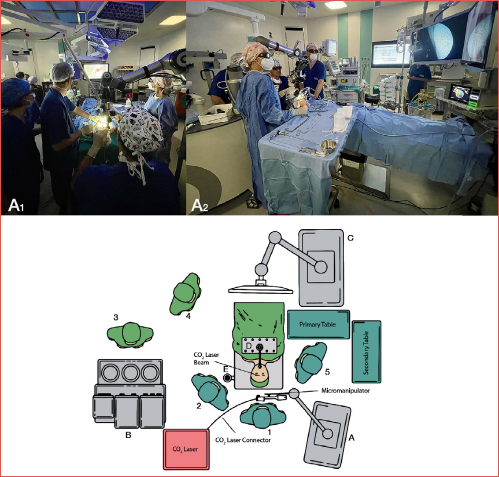
All procedures were carried out using the VITOM 3D exoscope (Karl Storz – Tuttlingen, Germany), which is equipped with a 3D-HD camera and the ARTip™ robotic cruise system. This integrated system can be controlled manually by dragging the robotic arm after the clutches are unlocked or using a controller joystick called “IMAGE 1 – Pilot” and a treadle. The exoscope features include zoom (2x to 30x magnification), focus, integrated illumination, and horizontal alignment. Moreover, the exoscope produces either 4K or 3D images, which are displayed on a 3D-HD screen. The processing of stereoscopic images is made possible by polarised glasses worn by the operators and OR staff.
Transoral exoscopic laryngeal surgery (TOELS)
Inclusion criteria for TOELS were: (1) early-intermediate suspicious or malignant lesions classified as cT1- cT2 N0 13 and (2) good predicted laryngeal exposure according to the “Laryngoscore” 14. Forty male patients with a mean age of 70.1 ± 10.8 years were included. Of these, 31 (77.5%) were affected by a glottic neoplasm, and 9 (22.5%) by a supraglottic tumour. The majority (25/31) of patients with glottic lesions were diagnosed at an early stage and 90% were treated by unilateral cordectomy.
According to our policy, the management of margin status after transoral laryngeal surgery was conducted as follows: one single superficial positive margin was subjected to a periodic endoscopic follow-up while multiple superficial positive margins or deep positive margins were managed with additional treatments, which were weighed on a case-by-case basis 15.
Laryngeal exposure was achieved by reproducing the Boice-Jackson position and using a range of laryngoscopes depending on the patient’s anatomy and the desired exposure.
Surgical procedures were classified according to the classification of cordectomies and endoscopic supraglottic laryngectomies proposed by the European Laryngological Society (Tab. I) 16-18.
The abovementioned exoscopic system was coupled with a CO2 laser Digital AcuBlade Micromanipulator (UltraPulse® DUO CO2 Surgical Laser, Lumenis, Yokneam, Israel), while all the surgical instruments employed during TOELS were the same as those used in standard transoral laryngeal microscopic surgery (TOLMS). During TOELS, the exoscope is positioned about 15 cm from the proximal opening of the laryngoscope and the surgeons are positioned behind the head of the patient, while the ARTip™ cruise system is located on the right side. It is recommended to place the 3D monitor above the patient’s legs, with an approximate distance from the surgeons of 1.50 m (Cover figure).
Transoral exoscopic oropharyngeal surgery (TOEOS)
Inclusion criteria for TOEOS were: (1) early-intermediate suspicious or malignant lesions classified as cT1, cT2 and selected cT3 N0-N+ 13 and (2) good predicted oropharyngeal exposure 14.
Fourteen patients with an average age of 70.8 ± 10.9 years affected by oropharyngeal squamous cell carcinoma (OPSCC) were enrolled. In most patients (54.6%), adequate exposure of the entire oropharynx was obtained with the Feyh-Kastenbauer Weinstein-O’Malley (FK-WO) retractor. Nine (69.2%) cases underwent concomitant neck dissection for the presence of suspicious neck lymph nodes. Surgical and demographic data are reported in Table II.
The surgical setting of TOEOS is the same as described for TOELS, although in this scenario there may be one or two assistant surgeons, who can easily help the first operator by standing on both sides of the patient’s head and hold other surgical instruments. Moreover, one assistant constantly arranges the framing and focusing of the image using the IMAGE 1 – Pilot and the treadle.
Results
TOELS
In most patients with a glottic lesion (25/31; 80.6%), the histopathological report showed the presence of SCC. TOELS achieved negative superficial and deep margins in most cases (80.8% and 76.9%, respectively), while multiple positive superficial margins were found in only one case (3.8%). In this latter patient a close endoscopic follow-up was performed since the patient refused any further treatment. Four of six patients with deep positive margins underwent re-excision, while two patients refused any intervention and were monitored with close endoscopic and radiologic follow-up. The final histopathology reports showed the absence of residual tumour in all cases, except for one where the resection margin resulted negative. As a result, 81.5% of patients with a glottic lesion achieved definitive negative margins.
Finally, 8 of 9 (92.8%) patients with supraglottic tumours ended their surgical treatment with a histopathological diagnosis of SCC and two (22.2%) had evidence of one deep and one superficial positive margin. Both were managed with a transoral microscopic re-excision. The final histopathology showed residual tumour with negative surgical margins in the first case, and a close positive deep margin in the second case. This latter patient underwent strict endoscopic-radiological follow-up. Finally, 8 of 9 (92.8%) TOEOS patients ended their surgical treatment plan with histological evidence of clear margins.
The mean operative time was 73.7 ± 35.9 minutes for the glottic group and 90.6 ± 33.4 minutes for the supraglottic group. Mean hospitalisation time was 2.5 ± 1 days and 8.8 ± 8.6 days for the glottic and supraglottic groups, respectively. No complications were reported. No tracheotomies were performed, and all patients were discharged with an oral diet. Data on the entire TOELS cohort are shown in Table I. Considering the limited follow-up, further oncological and survival outcomes were not assessable.
TOEOS
All patients were affected by SCC, while 50% of the cohort was positive for HPV infection. In only one case (7.7%) was a positive resection margin (deep) identified in the final histopathologic report. This patient underwent a widening of the previous surgical margin that resulted without residual tumour. Finally, TOEOS achieved a rate of negative surgical margins of 100%. The mean operative time was 140.3 ± 82.1 minutes, and the average hospitalisation time was 10.3 ± 3.8 days. Postoperative complications occurred in 2 patients (neck bleeding and sudden death due to cardiac causes), but neither was related to the transoral intervention. All patients were discharged with an oral diet and free from tracheotomy. Table II summarises data on the oropharyngeal population. Considering the limited follow-up, further oncological outcomes were not assessable.
Discussion
In recent years continuous progress has been made in novel surgical devices. The investigation of emerging technologies should be pursued from the point of view of patient outcomes. This means that, in choosing a therapy, or a surgical tool, it is necessary to not cause harm to the patient and for this reason, among the possible choices, the one that has the least side effects should be privileged.
Amidst the diffusion of new technology, it is important to consider its utility. Therefore, before jumping on the technology bandwagon, it is worthwhile to ask if the employment of exoscopes in our field is driven by technology or if its clinical use is spontaneously being driven by its technical advantages.
TOLMS is a well-established surgical approach and a viable alternative to open-neck approaches and radiotherapy in early-intermediate laryngeal cancer 13. The technique allows sound oncological results while preserving organ function and ensuring high salvage rates in case of persistent/recurrent or secondary laryngeal tumours.
This type of surgery is characterised by a narrow-margin approach, which makes the process of performing a safe and clean resection challenging. Moreover, the use of CO2 laser invariably leads to tissue coarctation and consequent margin shrinkage, which further hampers the possibility to obtain wide negative surgical margins 19. In addition, the success of the procedure cannot ignore the importance of laryngeal exposure.
As such, adequate exposure is a prerogative to obtain consistent oncological outcomes. In the current work, we were able to expose patients with comparable ease as in previous experiences, meaning that the surgical light through different laryngoscopes was not dissimilar by TOLMS or TOELS. Our findings are supported by the rates of superficial and deep negative margins, which are comparable to our past results and even to larger series 8,20,21. Lastly, in the era of cost restrictions, the hospital stay (2.5 ± 1 days for glottic and 8.6 ± 8.6 days for supraglottic cancers) was similar to the published literature 22, and thus TOELS did not impact hospitalisation time.
The advantage we encountered, mostly for supraglottic tumours, is the possibility to combine the benefits of TOLMS (optical magnification, tactile feedback, CO2 laser) with the superiority of TORS in terms of field of exposure, hot cutting devices, and multiple surgical arms. Moreover, the ability to use different types of lasers that can be coupled to the exoscope, with particular cutting characteristics, allowed to employ the ideal tool according to the need, without changing patient’s exposure or OR settings.
The optimal management of early-stage OPSCC with surgery or radiotherapy continues to be a matter of clinical debate 23. Among different surgical approaches, both TORS and TOLMS result in excellent functional and oncologic outcomes 2. Even if 1-2 mm margins are currently considered acceptable for glottic cancer, a survey of 476 head and neck surgeons considered 5 mm to be the definition of a close margin in oropharyngeal cancer 24, which is concordant with the National Comprehensive Cancer Network (NCCN) guidelines 25. In this light, the status of margins after TOEOS in our cohort was: 53.8% negative, 7.7% close superficial (< 5 mm), 30.8% close deep (< 5 mm), and 7.7% positive deep. Therefore, after re-intervention on the patient with positive margins, we obtained 78% of definitive negative margins, a percentage that is aligned with the reports of experienced robotic surgeons. Ten years ago, Hinni et al. evaluated patients undergoing TOLMS for OPSCC and reported average deep and peripheral margins of 1.98 mm and 2.98 mm, respectively 26. They additionally measured the magnetic resonance imaging-based dimensions of superior constrictor muscle in healthy patients, reporting a mean thickness of 2.4 mm at its thinnest portion. At this level, no additional fascial layer exists deep to the muscle, especially in the region of the inferior tonsillar fossa. Instead, only parapharyngeal fat is found at this point, which serves as a poor oncologic margin, meaning that wider surgical margins are often unobtainable. Accordingly, the ECOG 3311 risk stratification supports to some extent a close margin definition of 3 mm 27, which makes our TOEOS margins data coherent.
Second, the exoscope setup can be paired with several instruments with different cutting features (from monopolar Bovie to CO2 laser or diode laser, etc.), depending on tissue targets. Lastly, it allows four-hands procedures with proper suction, retraction, and haptic feedback.
In our experience, the main technical drawback of TOEOS is encountered in certain tongue base tumours at the bottom of the vallecula, where the surgical corridor from the exoscope, through the retractor, is tangential to the apex of the vallecula, creating a flat angle that is difficult to manage (Fig. 1).
The hospital stay (10.3 ± 3.8 days) of our patients falls within the range reported in the literature of 9-12 days 28. Similarly, the complication rate in our series (5.4%), even if not directly linked to the exoscopic procedure, is comparable to the literature, since several authors reported complications with TORS ranging from 10% 29 to 16.5% 30.
Overall, both the surgical (i.e. margin status after the resection) and the morbidity outcomes (complication rate, hospitalisation and surgical times) are in line with the current literature for transoral microsurgery procedures and in selected cases the exoscopic approach allowed the surgeons to perform a more accurate and precise resection, thanks to the abovementioned features.
Undoubtedly, even if we tried to select patients in the same way as we normally do for transoral microsurgical procedures, a selection bias still exists, and this must be acknowledged as a limit of the study. Similarly, both the retrospective and monocentric nature of the research and the low numerosity of the cohort represent limitations of our work, together with the absence of a transoral microscopic surgery control group to efficiently compare outcomes, as in a recent work by Piazza and coworkers 8. These limitations will be addressed in further comparative studies.
Conclusions
In head and neck surgery, the use of a 3D exoscope can offer potential benefits. It improves visualisation and magnification of the surgical site, which helps to increase the accuracy of the surgery. Moreover, it enables the use of multiple surgical instruments and lasers during four-hands procedures, leading to successful outcomes, without adding surgical complications. In addition, it may produce a better collaboration among the OR staff, since everyone can see the surgical field on 3D monitors. Further research involving larger patient populations and multiple centres are necessary to confirm these findings and evaluate the generalisability of our results.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
FM, EB, AIo, CS, GPe: study conception and design; EB, AIo: data collection; AIo, FM, CS, EB: analysis and interpretation of results; AIo, EB, FM: draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Ethical consideration
This study was approved by the Institutional Ethics Committee (CER Liguria: 230/2019). The research was conducted ethically, with all study procedures being performed in accordance with the requirements of the World Medical Association’s Declaration of Helsinki.
Written informed consent was obtained from each participant/patient for study participation and data publication.
History
Received: February 10, 2024
Accepted: June 27, 2024
Figures and tables
Figure 1. Endoscopic (left figure) and radiologic (right figure) views of a right tongue base tumour located in close relationship with the bottom of the vallecula. Managing these types of oropharyngeal tumours (marked as an asterisk) through a transoral exoscopic setup is complicated since the surgical corridor provided by the exoscope is tangential to the apex of the vallecula, thus creating a flat angle that is difficult to manage. BoT: base of tongue; E: epiglottis; V: vallecula.
| Variable | Value |
|---|---|
| Patients | 40 (100%) |
| Age | 70.1 ± 10.8 |
| Sex | |
| Male | 32 (80%) |
| Female | 8 (20%) |
| Anatomical site | |
| Glottis | 31 (77.5%) |
| Supraglottis | 9 (22.5%) |
| Clinical staging | |
| Glottic group | |
| cTis | 1 (3.8%) |
| cT1 | 19 (76%) |
| cT1a | 9 (47.4%) |
| cT1b | 10 (52.6%) |
| cT2 | 2 (7.7%) |
| cT3 | 4 (15.4%) |
| Supraglottic group | |
| cT1 | 3 (37.5%) |
| cT2 | 4 (50%) |
| cT3 | 1 (12.5%) |
| Surgical procedure | |
| Glottic group 17 | |
| Unilateral cordectomy | 28 (90.3%) |
| Type I | 4 (14.3%) |
| Type II | 15 (53.6%) |
| Type III | 3 (10.7%) |
| Type V | 6 (21.4%) |
| Type VI | 1 (3.6%) |
| Bilateral type II cordectomy | 2 (6.4%) |
| TOELS NOS | 1 (3.2%) |
| Supraglottic group 16 | |
| Endoscopic supraglottic laryngectomy | 3 (33.3%) |
| Type I | 2 (75%) |
| Type IIIa | 1 (25%) |
| Endoscopic supraglottic laryngectomy + selective neck dissection (level II-IV) | 5 (55.6%) |
| Type IIIb | 3 (60%) |
| Type IVb | 2 (40%) |
| TOELS NOS | 1 (11.1%) |
| Laryngeal exposure | |
| Glottic group | |
| Microfrance 121 laryngoscope | 24 (77.4%) |
| Hinni laryngoscope | 3 (9.7%) |
| Dedo laryngoscope | 2 (6.4%) |
| FK-WO retractor | 1 (3.2%) |
| Supraglottic group | |
| Hinni laryngoscope | 7 (77.8%) |
| Microfrance 121 laryngoscope | 2 (22.2%) |
| Laryngoscore 14 | |
| Glottic group | 4.6 ± 1.6 |
| Supraglottic group | 5 ± 2.4 |
| Definitive histology | |
| Squamous cell carcinoma | 33 (82.5%) |
| Cyst | 1 (2.5%) |
| Keratosis | 6 (15%) |
| Margin status – superficial margin | |
| Glottic group | |
| Negative | 21 (80.8%) |
| Single positive | 4 (15.4%) |
| Multiple positive | 1 (3.8%) |
| Supraglottic group | |
| Negative | 6 (77.8%) |
| Single positive | 2 (22.2%) |
| Operative time (minutes) | |
| Glottic group | 73.7 ± 35.9 |
| Supraglottic group | 90.6 ± 33.4 |
| Hospitalisation (days) | |
| Glottic group | 2.5 ± 1 |
| Supraglottic group | 8.6 ± 8.6 |
| NOS: not otherwise specified; FK-WO retractor: Feyh-Kastenbauer Weinstein-O’Malley retractor. | |
| Variable | Value |
|---|---|
| Patients | 14 (100%) |
| Age | 70.8 ± 10.9 |
| Sex | |
| Male | 3 (21.4%) |
| Female | 11 (78.6%) |
| Anatomical site | |
| Base of tongue | 5 (35.7%) |
| Tonsil – lateral wall of oropharynx | 8 (57.1%) |
| Posterior wall of oropharynx | 1 (7.2%) |
| Clinical staging | |
| Stage I | 6 (46.1%) |
| cT1N0 p16- | 2 (33.3%) |
| cT1N1 p16+ | 2 (33.3%) |
| rcT1N1 p16+ | 1 (16.6%) |
| cT2N0 p16+ | 1 (16.6%) |
| Stage III | 5 (38.5%) |
| cT3N0 p16- | 1 (20%) |
| cT3N0 p16+ | 1 (20%) |
| cT3N1 p16- | 2 (40%) |
| cT3N1 p16+ | 1 (20%) |
| Stage IVa | 1 (7.7%) |
| cT3N2b p16- | |
| Unknown primary (cTxN1) | 1 (7.7%) |
| Surgical procedure | |
| Transoral lateral oropharyngectomy | 7 (50%) |
| Base of tongue mucosectomy | 5 (35.6%) |
| Endoscopic supraglottic laryngectomy IVa | 1 (7.2%) |
| Posterior oropharyngeal wall mucosectomy | 1 (7.2%) |
| Oropharyngeal retractor | |
| Hinni laryngoscope | 5 (45.4%) |
| FK-WO retractor | 6 (54.6%) |
| Neck dissection | |
| Performed | 9 (69.2%) |
| Ipsilateral selective neck dissection (II-IV) | 8 (88.9%) |
| Bilateral selective neck dissection (II-IV) | 1 (11.1%) |
| Not performed | 4 (30.8%) |
| Margin status | |
| Negative | 7 (53.8%) |
| Close superficial | 1 (7.7%) |
| Close deep | 4 (30.8%) |
| Positive deep | 1 (7.7%) |
| Pathological staging | |
| Stage I | 7 (53.8%) |
| pT1cN0 p16- | 1 (14.3%) |
| pT1N0 p16+ | 1 (14.3%) |
| pT2N0 p16+ | 4 (57.1%) |
| pT2N1 p16+ | 1 (14.3%) |
| Stage II | 1 (7.7%) |
| pT2cN0 p16- | |
| Stage III | 4 (30.8%) |
| pT1N1 p16+ | 1 (25%) |
| pT3cN0 p16- | 1 (25%) |
| pT2N1 p16- | 1 (25%) |
| pT3N1 p16- | 1 (25%) |
| Stage IVa | 1 (7.7%) |
| pT3N2b p16- | |
| Operative time (minutes) | 140.3 ± 82.1 |
| Hospitalisation (days) | 10.3 ± 3.8 |
| Complications | 2 (15.4%) |
| Bleeding from neck vessel | 1 |
| Death (cardiac arrest) | 1 |
| FK-WO retractor: Feyh-Kastenbauer Weinstein-O’Malley retractor. | |
References
- Carobbio A, Missale F, Fragale M. Transoral laser microsurgery: feasibility of a new exoscopic HD-3D system coupled with free beam or fiber laser. Lasers Med Sci. 2021;36:1865-1872. doi:https://doi.org/10.1007/s10103-020-03221-w
- Cammaroto G, Stringa L, Zhang H. Alternative applications of trans-oral robotic surgery (TORS): a systematic review. J Clin Med. 2020;9. doi:https://doi.org/10.3390/jcm9010201
- Ferlito S, La Mantia I, Caruso S. High definition three-dimensional exoscope (VITOM 3D) in E.N.T. surgery: a systematic review of current experience. J Clin Med. 2022;11:1-20. doi:https://doi.org/10.3390/jcm11133639
- De Virgilio A, Costantino A, Mondello T. Pre-clinical experience with the VITOM 3D and the ARTip cruise system for micro-laryngeal surgery. Laryngoscope. 2021;131:136-138. doi:https://doi.org/10.1002/lary.28675
- Bartkowiak E, Łuczewski Ł, Chou J. Is the 3D exoscope better than the surgical microscope in parotid surgery: a prospective, randomized single-center study. Eur Arch Otorhinolaryngol. 2022;279:1029-1034. doi:https://doi.org/10.1007/s00405-021-06876-5
- Shirzadi A, Mukherjee D, Drazin D. Use of the video telescope operating monitor (VITOM) as an alternative to the operating microscope in spine surgery. Spine (Phila Pa 1976). 2012;37:1517-1523. doi:https://doi.org/10.1097/BRS.0b013e3182709cef
- Paderno A, Deganello A, Lancini D. Is the exoscope ready to replace the operative microscope in transoral surgery?. Curr Opin Otolaryngol Head Neck Surg. 2022;30:79-86. doi:https://doi.org/10.1097/MOO.0000000000000794
- Piazza C, Gennarini F, Montenegro C. Transoral laser exoscopic surgery of the larynx: state of the art and comparison with traditional transoral laser microsurgery. Acta Otorhinolaryngol Ital. 2024;44:S3-S11. doi:https://doi.org/10.14639/0392-100X-suppl.1-44-2024-N2850
- Singla A, Sahni D, Gupta A. Surgical anatomy of round window and its implications for cochlear implantation. Clin Anat. 2014;27:331-336. doi:https://doi.org/10.1002/ca.22339
- Rubini A, Di Gioia S, Marchioni D. 3D exoscopic surgery of lateral skull base. Eur Arch Otorhinolaryngol. 2019;277:687-694. doi:https://doi.org/10.1007/s00405-019-05736-7
- Piazza C, Del Bon F, Paderno A. The diagnostic value of narrow band imaging in different oral and oropharyngeal subsites. Eur Arch Otorhinolaryngol. 2016;273:3347-3353. doi:https://doi.org/10.1007/s00405-016-3925-5
- Lydiatt W, Patel S, Ridge J. AJCC Cancer Staging Manual. (Amin M, Edge S, Greene F, eds.). Springer Nature; 2017.
- Marchi F, Filauro M, Missale F. A multidisciplinary team guided approach to the management of cT3 laryngeal cancer: a retrospective analysis of 104 cases. Cancers (Basel). 2019;11. doi:https://doi.org/10.3390/cancers11050717
- Piazza C, Mangili S, Del Bon F. Preoperative clinical predictors of difficult laryngeal exposure for microlaryngoscopy: the Laryngoscore. Laryngoscope. 2014;124:2561-2567. doi:https://doi.org/10.1002/lary.24803
- Fiz I, Mazzola F, Fiz F. Impact of close and positive margins in transoral laser microsurgery for Tis-T2 glottic cancer. Front Oncol. 2017;7. doi:https://doi.org/10.3389/fonc.2017.00245
- Remacle M, Hantzakos A, Eckel H. Endoscopic supraglottic laryngectomy: a proposal for a classification by the working committee on nomenclature, European Laryngological Society. Eur Arch Otorhinolaryngol. 2009;266:993-998. doi:https://doi.org/10.1007/s00405-008-0901-8
- Remacle M, Eckel H, Antonelli A. Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. Eur Arch Otorhinolaryngol. 2000;257:227-231. doi:https://doi.org/10.1007/s004050050228
- Remacle M, Van Haverbeke C, Eckel H. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur Arch Otorhinolaryngol. 2007;264:499-504. doi:https://doi.org/10.1007/s00405-007-0279-z
- Mariani C, Carta F, Tatti M. Shrinkage of specimens after CO(2) laser cordectomy: an objective intraoperative evaluation. Eur Arch Otorhinolaryngol. 2021;278:1515-1521. doi:https://doi.org/10.1007/s00405-021-06625-8
- Blanch J, Vilaseca I, Bernal-Sprekelsen M. Prognostic significance of surgical margins in transoral CO2 laser microsurgery for T1-T4 pharyngo-laryngeal cancers. Eur Arch Otorhinolaryngol. 2007;264:1045-1051. doi:https://doi.org/10.1007/s00405-007-0320-2
- Puxeddu R, Piazza C, Mensi M. Carbon dioxide laser salvage surgery after radiotherapy failure in T1 and T2 glottic carcinoma. Otolaryngol Head Neck Surg. 2004;130:84-88. doi:https://doi.org/10.1016/j.otohns.2003.07.002
- Chiesa Estomba C, Reinoso F, Velasquez A. Complications in CO2 laser transoral microsurgery for larynx carcinomas. Int Arch Otorhinolaryngol. 2016;20:151-155. doi:https://doi.org/10.1055/s-0035-1569145
- Nichols A, Theurer J, Prisman E. Randomized trial of radiotherapy versus transoral robotic surgery for oropharyngeal squamous cell carcinoma: long-term results of the ORATOR Trial. J Clin Oncol. 2022;40:866-875. doi:https://doi.org/10.1200/JCO.21.01961
- Meier J, Oliver D, Varvares M. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck. 2005;27:952-958. doi:https://doi.org/10.1002/hed.20269
- Head and Neck Cancers (version 1.2022).
- Tomblinson C, Fletcher G, Hu L. Determination of posterolateral oropharyngeal wall thickness and the potential implications for transoral surgical margins in tonsil cancer. Head Neck. 2021;43:2185-2192. doi:https://doi.org/10.1002/hed.26693
- Ferris R, Flamand Y, Weinstein G. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: an ECOG-ACRIN Cancer Research Group Trial (E3311). J Clin Oncol. 2022;40:138-149. doi:https://doi.org/10.1200/JCO.21.01752
- Holsinger F, McWhorter A, Ménard M. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region: technique, complications, and functional results. Arch Otolaryngol Head Neck Surg. 2005;131:583-591. doi:https://doi.org/10.1001/archotol.131.7.583
- Lörincz B, Möckelmann N, Busch C. Functional outcomes, feasibility, and safety of resection of transoral robotic surgery: single-institution series of 35 consecutive cases of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Head Neck. 2015;37:1618-1624. doi:https://doi.org/10.1002/hed.23809
- Weinstein G, O’Malley B, Magnuson J. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. 2012;122:1701-1707. doi:https://doi.org/10.1002/lary.23294
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2024 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1156 times
- PDF downloaded - 233 times




