Rhinology
Vol. 45: Issue 5 - October 2025
Optimising care for chronic rhinosinusitis with nasal polyps and asthma in multimorbid patients: a multidisciplinary Delphi consensus
Abstract
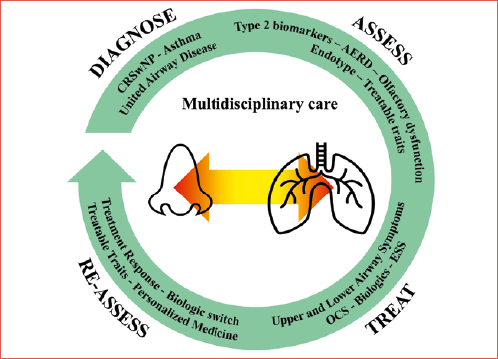
Objective. Chronic rhinosinusitis with nasal polyps (CRSwNP) and asthma often coexist and share common underlying pathophysiological mechanisms. This study addresses challenges in the diagnosis and treatment of multimorbid patients with CRSwNP and asthma providing new insights for their management.
Methods. Using a modified Delphi method, a scientific board of 40 Italian clinicians (pulmonologists, allergists/clinical immunologists, and ear, nose, and throat specialists) developed consensus statements regarding the multimorbid patient with severe CRSwNP and asthma and including the disease burden, assessment, treatment and multidisciplinary management of multimorbid patients.
Results. All statements reached consensus. Experts acknowledged the substantial disease burden, the key pathophysiological role of type 2 inflammation in multimorbid patients and the need for multidisciplinary care. The use of biologics represents a highly effective therapeutic opportunity for patients with comorbidities, optimising overall outcomes. Integrated care pathways and regular multidisciplinary reassessment were deemed essential for treatment decisions and improved care in multimorbid patients.
Conclusions. This consensus highlights the value of a multidisciplinary approach for multimorbid CRSwNP and asthma patients, advocating for endotype-driven therapies and standardised pathways in clinical practice.
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a complex condition characterised by symptoms that profoundly impact quality of life (QoL) 1-3. CRSwNP is frequently accompanied by comorbidities, particularly those affecting the lower airways; among these, asthma is present in up to 67% of patients with CRSwNP. Similarly, CRSwNP is often found in patients with asthma, with a stronger association observed in those with severe asthma (57.1-62%) compared to mild asthma (38-42.9%). When CRSwNP and asthma coexist in the same patient, both diseases manifest with more severe symptoms compared to patients with either condition alone 4. Given the strong pathophysiological interconnection between CRSwNP and asthma and, more generally, between upper and lower respiratory tract disorders, the “united airway disease” theory has gained increasing recognition 5.
Type 2 (T2) inflammatory mechanism is a common underlying cause of CRSwNP and asthma in multimorbid patients 6. In patients with severe asthma who also exhibit substantial sinus disease, analysis of computed tomography (CT) scans has revealed a positive association between mucosal thickening and elevated T2 inflammatory markers, such as eosinophil counts in sputum and blood, and high fractional exhaled nitric oxide (FeNO) levels 7. This shared T2 mechanism is primarily triggered by epithelial-targeting stimuli, such as allergens, superantigens, and pathogens. These stimuli compromise epithelial barrier integrity, leading to dysfunction that facilitates downstream inflammatory pathway activation and perpetuates chronic inflammation in the upper and lower airways – a hallmark of both CRSwNP and asthma 8,9. In turn, the damaged epithelium releases alarmin cytokines such as thymic stromal lymphopoietin (TSLP), IL-33 and IL-25, which amplify downstream T2 inflammatory pathways by promoting the secretion of IL-4, IL-5, and IL-13 in airway diseases 10. Intriguingly, the severity of asthma and CRSwNP and the extent of T2 inflammatory responses are most pronounced in patients with nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD) compared to those without 11.
Concerning clinical management, guidelines for asthma and CRSwNP both recommend the importance of an objective diagnosis supported by instrumental examinations and stress the need to measure disease severity and reassess disease control to guide treatment decisions. Recommended background therapies include local corticosteroids for both conditions (inhaled and intranasal for asthma and CRSwNP, respectively), whereas systemic corticosteroids should be avoided 2,12. To date, three biologic drugs have been approved as add-on therapies for severe, uncontrolled CRSwNP (dupilumab, omalizumab, mepolizumab), and other monoclonal antibodies (mAbs) (depemokimab, tezepelumab, itepekimab, lebrikizumab) are either in preclinical development or under evaluation in ongoing clinical trials. The advent of biologics for the treatment of CRSwNP has led to a redefinition of treatment goals and measures of response in this disease 13. By targeting distinct pathogenic drivers of T2 inflammation, these pharmacological agents represent innovative immunomodulating options for both CRSwNP and asthma in multimorbid patients 14.
For many years, the nose and lungs have been assessed and treated as separate entities in clinical practice. However, the united airway disease theory emphasises the need to evaluate the upper and lower respiratory tracts as a single system for several reasons: anatomical and physiological continuity, shared inflammatory pathways, impact on disease severity and control, coexistence of diseases, shared risk factors, improved diagnosis and treatment outcomes, exacerbation and disease progression, discordant response to the same treatment (for example using biologics) 15. Therefore, a multidisciplinary team should be involved at all stages of care – from diagnosis to planning of treatment and follow-up – to achieve optimal clinical outcomes in both diseases. This team should include allergists/clinical immunologists, pulmonologists, and ear, nose, and throat (ENT) specialists, with a solid understanding of inflammatory pathogenic mechanisms and expertise in novel treatment 16. As mentioned above, the use of mAbs against key T2 inflammatory players may promote a unified approach to address difficult-to-treat cases with coexisting CRSwNP and asthma. With the growing adoption of biologics for the treatment of severe, uncontrolled CRSwNP, the multidisciplinary management of multimorbid patients is becoming increasingly essential in clinical practice, especially at specialised referral centres 17.
In this study, a scientific board consisting of pulmonologists, allergologists/clinical immunologists, and ENT specialists discussed gaps and challenges in the diagnosis and treatment of patients with CRSwNP and asthma, with a focus on severe CRSwNP manifestation. Using a modified Delphi methodology, the board developed 20 statements, guiding towards an improved multidisciplinary care model that enhances communication and knowledge exchange among specialties, while prioritising the individual needs of multimorbid patients.
Materials and methods
The scientific board included 40 members, including 10 pulmonologists, 13 allergist-clinical immunologists and 17 ENT specialists, ensuring the comprehensive collection of expert perspectives from the three key specialties most involved in the care of patients with both CRSwNP and asthma in Italy. The initiative was supported and promoted by the Rhinology Italian Committee of the Italian Society of Otorhinolaryngology and by the Italian Association of Hospital Pulmonologists (AIPO).
The consensus procedure followed a modified Delphi method, which consists of multiple rounds of anonymous assessment and rating of statements previously developed by a group of experts. This method is widely recognised for achieving consensus in a specific field to help define best practices and offer practical guidance for clinical practice. Given its proven efficacy, this approach was chosen to gather insights among experts and address gaps and unmet needs in the management of multimorbid patients.
The main topics discussed by the scientific board were: 1) disease burden in multimorbid patients with severe CRSwNP and asthma, 2) assessment of multimorbidity, 3) need for multidisciplinary management for CRSwNP and asthma patients, and 4) treatment and re-assessment of multimorbid patients. The scientific board was divided into four distinct groups, each assigned to one of the four identified topics. Each group was responsible for conducting a thorough literature review and producing statements related to their designated topic. Comprehensive searches were performed across key medical databases, including MEDLINE/PubMed, EMBASE, the Cochrane Library, and DB Central. Finally, a total of 20 statements were formulated.
The statements were voted by the scientific board using a dedicated online platform: SurveyMonkey (SurveyMonkey Inc., San Mateo, California, USA, www.surveymonkey.com.); two rounds of voting took place between October 2023 and May 2024. The voting system clinicians were asked to rate their agreement level with the 20 proposed statements using a 9-point Likert scale, with each number corresponding to a level of agreement/disagreement [from 1 to 3: disagree; from 4 to 6: neutral (nor agree nor disagree); from 7 to 9: agree]. Consensus was achieved when at least 70% of voters agreed with the statement. The first-round votes were analysed, presented and discussed virtually by the scientific board, and some statements amended if deemed necessary. Specifically, statements were reformulated based on specific criteria: (a) ambiguous responses or widely dispersed scores, (b) overly generic or difficult-to-understand phrasing, or (c) controversy arising from statements combining two distinct declarations, which were then divided into separate statements. For statements reporting specific values (e.g., cut-offs), sources from which these values were derived were provided. The revised statements were subsequently rated again using the same 9-point numeric scale during a second voting round, during which a new question was introduced to identify each member’s specialty, facilitating stratified data analysis.
Results
All 40 members of the scientific board voted on all the 20 statements. Overall, the consensus (agreement > 70% of voters) was reached for all statements after the second round of voting.
Disease burden in multimorbid patients with severe CRSwNP and asthma
Table I shows the consensus achieved for statements addressing the disease burden in multimorbid patients. Experts largely agreed on the elevated disease burden of multimorbid patients, with 100% of voters acknowledging that patients with both severe CRSwNP and asthma show greater clinical severity compared to patients with either condition alone (statement #1). The scientific board also agreed on the higher CRSwNP burden in patients with asthma who also present NSAID-ERD (statement #2, 97.5%), and the frequent need for increased doses of oral corticosteroids (OCS) per year in patients with CRSwNP and asthma compared to patients with CRSwNP alone (statement #3, 97.5%). Overall, 92.5% of experts concurred that severe, uncontrolled CRSwNP increases the risk of developing uncontrolled asthma (statement #4), with the extent of T2 inflammation contributing to the severity of symptoms and inducing structural changes in both the nose and lungs (statement #5).
Assessment of multimorbidity
Table II summarises the consensus reached on statements regarding the assessment of CRSwNP and asthma. The experts agreed that objective tests should be used to diagnose asthma in patients with CRSwNP who present indicative symptoms (statement #6, 97.5%); more in detail, 100% of experts confirmed that lower airway symptoms should suggest the presence of asthma, especially in patients with adult-onset, severe CRSwNP, high blood eosinophil count and aspirin intolerance (statement #7). Conversely, patients with asthma who experience persistent nasal symptoms are likely to have CRSwNP, particularly when these symptoms are accompanied by smell dysfunction (statement #8, 92.5%). In addition, the coexistence of CRSwNP and asthma is unanimously considered a strong clinical indicator of T2 inflammation (statement #9, 100%). Finally, the experts concurred that both historical and current levels of T2 biomarkers should be evaluated while considering the potential effect of concomitant treatments (e.g., OCS, biologics and others) (statement #10, 97.5%).
The need for multidisciplinary management for asthma and CRSwNP patients
The scientific board extensively discussed the ideal multidisciplinary diagnostic-therapeutic pathway for multimorbid patients; statements are shown in Table III. A total of 97.5% of voters agreed that a multidisciplinary team should determine the treatment approach for patients with CRSwNP and asthma. Various factors should be taken into account, including the severity and control of both diseases, the underlying endotype, clinical history, response to prior treatments, drivers of disease severity, and patient preferences (statement #11). A multidisciplinary assessment of patients is also deemed essential to rule out the presence of eosinophilic granulomatosis with polyangiitis (EGPA) in patients with severe CRSwNP and severe asthma who show blood eosinophilia higher than 1500 cells/mm3 (statement #12, 100%). In general, experts concurred that treatments should aim to achieve control of both diseases (statement #13, 97.5%); as such, treatment response should be evaluated by assessing clinical outcomes in both upper and lower airways (statement #15, 92.5%). Ideally, patients with CRSwNP and asthma should be assessed concomitantly by a multidisciplinary team composed of ENT specialists, pulmonologists and allergologists/clinical immunologists; if not feasible, experts encourage the implementation of a standardised referral pathway within the healthcare system for multimorbid patients (statement #14, 92.5%).
Treatment and re-assessment of multimorbid patients
Table IV shows the statements addressing treatment options and re-assessment of multimorbid patients. In total, 77.5% of experts agreed that biologics should be considered the initial therapeutic option in patients with severe CRSwNP and asthma who exhibit predictive factors for poor outcomes after endoscopic sinus surgery (ESS), such as elevated T2 biomarkers, NSAID-ERD, and poor asthma control (statement #16). The same percentage of voters confirmed that ESS should be adopted in patients with severe CRSwNP and asthma during treatment with biologics in case of poor control of sino-nasal symptoms, provided that it is performed after at least 6 months from the start of the biologic therapy (statement #17). In cases of persistent symptoms after ESS, several factors should be evaluated as potential causes of surgical failure, including the adequacy of the procedure, adherence to corticosteroid treatment, ongoing exposure to triggers, and a thorough analysis of the disease endotype (statement #18, 97.5%); in addition, the patient’s treatable traits should also be assessed (statement #19, 95%). The experts also concurred that the multidisciplinary team needs to discuss the switch from one biologic to another, and the choice should be based on the primary driver of severity (statement #20, 95%).
Discussion
The management of multimorbid patients is complex and requires clear recommendations and a standardised framework. Current guidelines for CRSwNP and asthma are predominantly disease-oriented rather than patient-oriented and lack a structured multidisciplinary strategy 2,12,18. To develop a multidisciplinary, patient-centred approach for multimorbid patients, a shift from viewing CRSwNP and asthma as isolated entities to recognising them as part of a unified airway disease is necessary. Today, the management of CRSwNP has evolved from unreliable phenotyping to a more accurate endotype-based classification 19. Consequently, clinicians can identify the underlying pathological mechanisms driving CRSwNP, which often overlap with those affecting coexisting conditions like asthma. By fostering communication and knowledge sharing among allergists/clinical immunologists, pulmonologists, and ENT specialists, multidisciplinary teams can develop comprehensive treatment plans that include innovative therapies to achieve superior control of both diseases. Previously, an Italian multidisciplinary consensus was conducted to support specialists in managing patients with CRSwNP with biologics 3. In this study, consensus statements provide a pragmatic framework for integrated management of severe CRSwNP and asthma, emphasising multidisciplinary care, detailed assessment, and the use of biologics to improve multimorbid patient care in clinical practice.
The scientific board acknowledges the substantial disease burden and clinical challenges related to coexisting CRSwNP and asthma, highlighting the bidirectional relationship between the two conditions and their close connection to T2 inflammation. CRSwNP with multimorbid asthma is associated with more severe sinonasal symptoms and worse QoL, and is more difficult to treat both medically and surgically, with an increased risk of post-surgical recurrence 4,20. Asthma is more difficult to control in the presence of CRSwNP, with multimorbid patients having more frequent exacerbations, greater airway obstruction and increased inflammatory markers compared to those without CRSwNP 21,22. Thus, patients suffering from both CRSwNP and asthma often rely on higher OCS usage compared to those with either condition alone and more at risk of steroid related morbidity and burden 23. Clinical manifestations are likely to become more severe if multimorbid patients also present NSAID-ERD; indeed, NSAID-ERD further exacerbates symptoms of both CRSwNP and asthma. Given the association between NSAID-ERD and severe eosinophilic inflammation in the upper and lower airways, the combination of CRSwNP, NSAID-ERD and asthma represents the clinical phenotype that most strongly demonstrates the interconnection between T2 inflammation and the severity of united airway disease 11. Importantly, T2 inflammatory pathways not only increase the severity of symptoms, but also contribute to structural changes in nasal and lung tissues over time. Increasing evidence shows that both upper and lower airways can respond to T2 inflammatory cytokines by activating remodelling processes, resulting in epithelial disruption, goblet cell hyperplasia, subepithelial fibrosis, smooth muscle thickening, and increased vascularisation in asthma, and stromal oedema, collagen/fibrin deposition, and polyp formation in CRSwNP 24,25,.
The scientific board emphasises the importance of using objective diagnostic tests to ascertain the presence of asthma and/or CRSwNP in patients already diagnosed with either CRSwNP or asthma, especially if they present elevated T2 biomarkers and/or NSAID-ERD. Asthma can be difficult to diagnose due to its heterogeneous and accessional nature. Patients with severe CRSwNP may underestimate their asthma symptoms due to the higher burden of nasal symptoms, leading to a high rate of asthma underdiagnosis 26. Strikingly, between 28% and 40% of patients with CRSwNP who underwent bronchial hyperresponsiveness evaluation were found to have previously undiagnosed asthma 4. Conversely, asthmatic patients complaining about persistent nasal symptoms, particularly smell dysfunction, should undergo diagnostic evaluation for CRSwNP. Most CRSwNP patients complain about hyposmia or anosmia; in addition, olfactory dysfunction is also indicative of an underlying T2 inflammatory endotype, with the severity of loss of smell being positively associated with nasal eosinophilia and elevated levels of IL-2, IL-5, IL-6, IL-10, IL-13, and immunoglobulin (Ig)E 27.
The experts widely agreed that both recent and historical levels of T2 biomarkers should be evaluated in multimorbid patients, taking into account the potential impact of concomitant treatments. Historical biomarker levels, measured in the absence of treatment, are likely to provide a more accurate representation of the underlying inflammatory signature than recent levels, which may be influenced by ongoing treatment and could appear within the normal range. This practical approach ensures a deeper understanding of the evolving inflammatory profile and is pivotal to guiding clinicians towards more effective and personalised treatment strategies.
The scientific board reiterates the key role of multidisciplinarity in effectively managing multimorbid patients. While ENT, pulmonology, and allergy/clinical immunology specialists should collaboratively manage all multimorbid patients, an in-depth multidisciplinary evaluation is especially paramount for difficult-to-treat or complex cases, such as multimorbid patients with NSAID-ERD and those exhibiting distinctive features suggestive of additional concomitant diseases, such as blood hypereosinophilia which could imply the diagnosis (EGPA). Specialists should cooperate to select the best treatment option to achieve control of the overall disease and should periodically re-assess patients to verify clinical outcomes in both the upper and lower airways.
According to the European Position Paper on Rhinosinusitis and Nasal Polyps 2020, biologics should be considered in patients with CRSwNP after undergoing ESS, unless they are not fit for surgery 2. Importantly, ESS may fail in long term control of symptoms in severe CRSwNP. In certain cases - such as patients with asthma, NSAID-ERD, or recurrence within 3 years of surgery – a pharmacological approach may be preferred over revision surgery and could be prioritised based on individual patient characteristics and needs 18,28,29. In a recent Delphi study, experts were uncertain about the use of biologics before ESS in multimorbid patients 30. In this consensus, most experts advocated for biologic-based therapy as the first-line treatment in patients with CRSwNP, poorly controlled asthma and a high risk of poor control after surgery. To date, omalizumab, mepolizumab and dupilumab are the mAbs targeting T2 inflammatory players that received approval for both severe CRSwNP and severe asthma. All have shown beneficial effects in both conditions, with the potential to drastically alter the disease trajectories. In addition, they significantly reduced the need for surgery as shown in randomised clinical trials, and the efficacy of dupilumab is independent of surgery history 31. These data highlight the potential to prioritise biologics over first-time surgery as their use can simultaneously improve outcomes in both diseases.
In case the biologic therapy only partially improves sinonasal symptoms, ESS should be performed after at least 6 months from the start of the biologic therapy, allowing sufficient time for a potential late response to the biologic therapy. A thorough investigation to understand the cause/s of the lack of control of sinonasal symptoms with biologic treatment and/or ESS should be carried out. Switching biologics in multimorbid patients should be carefully discussed by the multidisciplinary team, guided by a re-assessment of the patient’s endotype and treatable traits and further decisions should consider the pathology which is the driver of severity. The “treatable trait” approach is particularly valuable in the classification and management of united airway disease, allowing clinicians to select personalised therapies that are tailored to the patient’s unique disease-associated characteristics 32,33. Despite the promising response to biologic drugs, in a small group of patients a dissociation of response between upper and lower airways may be observed. This represents, for the panellists, an additional reason why multidisciplinary management is necessary 15. Of note, the arsenal of biologics for treating CRSwNP and asthma is rapidly expanding, with new therapies in development that promise to shape the future of personalised medicine in united airway disease by further improving clinical outcomes and QoL for multimorbid patients 14.
While the authors of this board believe that a multidisciplinary approach for complex conditions like CRSwNP and asthma is essential, they also acknowledge that its implementation in routine clinical practice presents several challenges. One major issue is the lack of integrated shared platforms. If electronic health record (EHR) systems are not standardised and widely available, sharing patient information and treatment plans becomes difficult. Coordinating appointments among multiple specialists can also be logistically difficult, leading to delays in care and increased patient burden. The need for more frequent visits to different specialists can also be problematic for patients, particularly those with limited mobility, transportation issues, or socioeconomic constraints. On the other hand, busy clinical schedules may restrict the time that specialists can spend on joint consultations or communication, compromising the quality of integrated care. Furthermore, different treatment philosophies or priorities among specialists may preclude an agreement on the optimal course of action for the patient. In particular, specialists may not fully appreciate the nuances of other disciplines involved in patient care, missing opportunities for holistic management. Without established guidelines or protocols for multidisciplinary care, each team member may approach the patient’s management differently, leading to inconsistencies in treatment plans.
The strengths of this Delphi consensus are the involvement of a large, multidisciplinary board of Italian specialists with extensive expertise in managing CRSwNP and asthma, and the pragmatic nature of the statements that complement existing guidelines to provide actionable guidance and address challenges in the clinical practice. The main limitation is the lack of an external expert panel beyond the scientific board, which could have provided additional perspectives and further validated the consensus findings.
Conclusions
A multidisciplinary approach is widely recognised for the diagnosis and treatment of multimorbid patients with upper and lower airway conditions, as it offers the potential for improved patient outcomes. However, most studies still consider asthma and CRSwNP as separate conditions rather than a single disease; in turn, clinicians face several challenges in managing multimorbid patients and many issues need to be addressed to implement a multidisciplinary approach in the clinical practice. Although more evidence is required to adequately inform on best practices, the consensus-based recommendations proposed in this and other studies are a first step toward establishing a standardised, patient-centred approach. By promoting collaboration among specialists and leveraging an endotype-driven framework, these recommendations support integrated care strategies for patients with CRSwNP and asthma and, more broadly, with united airway disease.
Acknowledgements
The initiative was supported and promoted by the Rhinology Italian Committee of the Italian Society of Otorhinolaryngology and cervico-facial surgery (SioChCf) and by the Italian Association of Hospital Pulmonologists (AIPO).
Conflict of interest statement
EDC, CC, VS: lecture fees and participations in experts board meeting of GSK, Novartis, Sanofi, Astrazeneca; CP: lecture fees and participations in experts board meeting of GSK, Novartis, Sanofi, Firma, Deca; EC: lecture fees and participations in experts board meeting of GSK, Novartis, Sanofi, NOOS; ILM: lecture fees and participations in experts board meeting of GSK, Novartis, Sanofi, Chiesi; FRMC, MC, SG, FP, JWS, MG: lecture fees from Sanofi and GSK; GO: lecture fees and participations in experts board meeting of GSK, Novartis, Sanofi; GLF: lecture fees and participations in experts board meeting of Astrazeneca, Sanofi;AM: lecture fee of Sanofi; FM: has received research grants from AstraZeneca, Sanofi, GSK, Chiesi Farmaceutici and lecture fees and advisory board fees from AstraZeneca, Chiesi Farmaceutici, GSK, Lusofarmaco; GEC: has received research grants lecture fees and advisory board fees from AstraZeneca, BI, Menarini, Lusofarmaco, Chiesi, Glaxo-Smith-Kline, Guidotti, Sanofi; FDM: has received research grants lecture fees and advisory board fees from AstraZeneca, BI, Menarini, Neopharmed Gentili, Zambon, Chiesi, Glaxo-Smith-Kline, Guidotti, MSD, Sanofi; EN: lecture fees and participations in experts board meeting of GSK, Novartis, Sanofi, Astrazeneca. Chiesi, Firma, Abbvie, LEO-Pharma. GWC reports research or clinical trials grants paid to his Institution from Menarini, AstraZeneca, GSK, Sanofi Genzyme and fees for lectures or advisory board participation from Menarini, AstraZeneca, CellTrion, Chiesi, Faes Farma, Firma, Genentech, Guidotti, GSK, HAL Allergy, Innovacaremd, Novartis, OM-Pharma, Red Maple, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes-Greer and Uriach Pharma; AD: lecture fees and participations in experts board meeting of Glaxo Smith Kline, Novartis, Sanofi, Astra Zeneca; MBB: has received lecture fees and/or advisory board fees from AstraZeneca, Menarini, Chiesi, Glaxo-Smith-Kline, Sanofi; SDG: lecture fees and participation in expert board meeting of Chiesi, AstraZeneca, GSK, Novartis, Sanofi; lecture fees: DBV, Stallergenes. CM received fees as a speaker in national and international congresses from Astrazeneca, Sanofi, GSK, Chiesi, Menarini, Guidotti, Lusofarmaco, Berlin Chemie, Zambon, Boehringher; EP, JG, GP, DM, GM, CC: no conflict of interest; MC: has received fees from AstraZeneca for serving on advisory boards and speaker fees from GSK and Sanofi; MD: lecture fees and participations in experts board meeting of GSK, Sanofi, Astrazeneca, Chiesi; GCP, FMP: lecture fee per Sanofi, DECA e GSK; AV: received lecture fees and advisory board fees from Sanofi, AstraZeneca, Glaxo-Smith-Kline, Firma; AM: received lecture fees and advisory board fees from Sanofi, AstraZeneca, Glaxo-Smith-Kline, Novartis, Chiesi; DB: received lecture fees from AstraZeneca, Chiesi, Glaxo Smith Kline, Guidotti, Grifols, Menarini, Novartis AG, Sanofi; MB,MC: has nothing to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
EDC, AV: conception and design of the study, acquisition of the data, analysis, and interpretation of the data; drafted the article and revised it for important intellectual content; gave final approval of the version to be submitted; agree to be accountable for all aspects of the work; MC, CP: acquisition of the data, analysis, and interpretation of the data; first drafted of the article, final approval of the manuscript; AV, AM, EC, GS, MC, FRC, CL, AD, GWC, GWS, MDA, MAC, MG, FAS, DM, VR, AM, EP, MC, SG, GP, CC, GEC, EH, EN, MBB, VS, GO, CM, SDG, CC, DB, MB, FM, GCP, GM, FDM, FP, JG: Delphi participation, gave final approval of the version to be submitted; agree to be accountable for all aspects of the work.
Ethical consideration
Not applicable.
History
Received: January 14, 2025
Accepted: March 22, 2025
Figures and tables
| No. | Statements | Response rate | Disagree | Neutral | Agree |
|---|---|---|---|---|---|
| n/N (%) | (1-3) | (4-6) | (7-9) | ||
| 1 | Patients with severe CRSwNP and asthma are characterised by greater clinical severity compared to patients with either condition alone | 40/40 (100%) | 0% | 0% | 100% |
| 2 | Patients with asthma and nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD), have a high likelihood of experiencing difficult-to-treat CRSwNP | 40/40 (100%) | 0% | 2.5% | 97.5% |
| 3 | Patients with severe CRSwNP and asthma often require higher annual doses of systemic steroids compared to patients with CRSwNP alone | 40/40 (100%) | 0% | 2.5% | 97.5% |
| 4 | Severe, uncontrolled CRSwNP contributes to the risk of uncontrolled asthma by increasing the risk of asthma exacerbations | 40/40 (100%) | 2.5% | 5% | 92.5% |
| 5 | In patients with severe CRSwNP and asthma, T2 inflammatory pathways are not only responsible for severe symptoms, but also for relevant structural changes in both lower and upper airways | 40/40 (100%) | 0% | 7.5% | 92.5% |
| No. | Statements | Response rate | Disagree | Neutral | Agree |
|---|---|---|---|---|---|
| n/N (%) | (1-3) | (4-6) | (7-9) | ||
| 6 | If asthma is suspected in patients with severe CRSwNP due to the presence of suggestive symptoms, objective tests must be used to confirm the diagnosis | 40/40 (100%) | 0% | 2.5% | 97.5% |
| 7 | Lower airway symptoms in patients with severe CRSwNP are highly suggestive of bronchial asthma, especially in case of adult-onset disease, blood eosinophilia and aspirin intolerance | 40/40 (100%) | 0% | 0% | 100% |
| 8 | Persistent nasal symptoms in asthmatic patients strongly suggest the presence of CRSwNP, especially when accompanied by smell dysfunction | 40/40 (100%) | 0% | 7.5% | 92.5% |
| 9 | The coexistence of asthma and CRSwNP is a strong clinical indicator of T2 inflammation | 40/40 (100%) | 0% | 0% | 100% |
| 10 | When assessing T2 biomarkers (e.g., blood or local eosinophilia, FeNO, total and specific IgE) in patients with severe CRSwNP and asthma, it is advisable to check recent and historical values, taking into account the potential impact of concomitant medical treatment (e.g., systemic steroids, biologics, anti-leukotrienes, etc.) | 40/40 (100%) | 0% | 2.5% | 97.5% |
| No. | Statements | Response rate | Disagree | Neutral | Agree |
|---|---|---|---|---|---|
| n/N (%) | (1-3) | (4-6) | (7-9) | ||
| 11 | In patients with CRSwNP and asthma, the choice of treatment must be carefully evaluated within a multidisciplinary team by assessing the severity and control of the two diseases, endotype, clinical history, response to previous treatments, drivers of severity and patient preference | 40/40 (100%) | 0% | 2.5% | 97.5% |
| 12 | In the presence of coexisting difficult-to-treat CRSwNP, severe asthma and blood hypereosinophilia (> 1500 cells/mm3), eosinophilic granulomatosis with polyangiitis (EGPA) should be always ruled out through a multidisciplinary assessment, both at diagnosis and during the follow-up | 40/40 (100%) | 0% | 0% | 100% |
| 13 | In patients with severe CRSwNP and asthma, therapeutic interventions aim to achieve disease control in both the upper and lower airways | 40/40 (100%) | 0% | 2.5% | 97.5% |
| 14 | Patients with CRSwNP and asthma should be ideally evaluated simultaneously by ENT, pulmonology, and allergy/clinical immunology specialists. If this is not feasible, a standardised referral pathway should be implemented within the healthcare system | 40/40 (100%) | 5% | 2.5% | 92.5% |
| 15 | In patients with severe CRSwNP and asthma, treatment response is assessed by assessing disease outcomes in both upper and lower airways | 40/40 (100%) | 0% | 0% | 100% |
| No. | Statements | Response rate | Disagree | Partially agree | Agree |
|---|---|---|---|---|---|
| n/N (%) | (1-3) | (4-6) | (7-9) | ||
| 16 | Biologics should be considered as the initial therapeutic option in patients with severe CRSwNP and asthma who exhibit predictive factors for poor surgical outcomes (e.g., markers of high T2 inflammatory response, NSAID-ERD, poor control of asthma symptoms) | 40/40 (100%) | 12.5% | 10% | 77.5% |
| 17 | In patients with severe CRSwNP and asthma who present poor control of sino-nasal symptoms despite biologic therapy, ESS may be considered a useful intervention to improve CRSwNP control, provided that it is performed at least 6 months after starting the biologic treatment | 40/40 (100%) | 0% | 22.5% | 77.5% |
| 18 | If patients with CRSwNP and concomitant asthma experience persistent poor symptom control even after ESS, potential reasons for failure need to be investigated, including adequacy of surgery, adherence to topical corticosteroids, continuous exposure to triggers, analysis of the disease endotype | 40/40 (100%) | 0% | 2.5% | 97.5% |
| 19 | In case of inadequate sino-nasal symptoms control in patients with CRSwNP and asthma, patients’ traitable traits should be investigated before switching from one biologic to another. | 40/40 (100%) | 2.5% | 2.5% | 95% |
| 20 | In patients with severe CRSwNP and asthma, the switch from one biologic to another needs to be discussed in a multidisciplinary setting and should consider the primary driver of severity | 40/40 (100%) | 0% | 5% | 95% |
References
- Chen S, Zhou A, Emmanuel B. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr Med Res Opin. 2020;36:1897-1911. doi:https://doi.org/10.1080/03007995.2020.1815682
- Fokkens W, Lund V, Hopkins C. European Position Paper on rhinosinusitis and nasal polyps 2020. Rhinology. Published online 2020:1-464. doi:https://doi.org/10.4193/Rhin20.600
- De Corso E, Pipolo C, Caminati M. Multidisciplinary decision-making-ITAlian Consensus after two years of real practice on the management of severe uncontrolled CRSwNP by biologics (ITACA Study). Curr Allergy Asthma Rep. 2024;24:143-154. doi:https://doi.org/10.1007/s11882-024-01135-z
- Laidlaw T, Mullol J, Woessner K. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9:1133-1141. doi:https://doi.org/10.1016/j.jaip.2020.09.063
- Kanda A, Kobayashi Y, Asako M. Regulation of interaction between the upper and lower airways in united airway disease. Med Sci. 2019;7. doi:https://doi.org/10.3390/medsci7020027
- Khan A, Gouia I, Kamat S. Prevalence and severity distribution of type 2 inflammation-related comorbidities among patients with asthma, chronic rhinosinusitis with nasal polyps, and atopic dermatitis. Lung. 2023;201:57-63. doi:https://doi.org/10.1007/s00408-023-00603-z
- ten Brinke A, Grootendorst D, Schmidt J. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621-626. doi:https://doi.org/10.1067/mai.2002.122458
- Wynne M, Atkinson C, Schlosser R. Contribution of epithelial cell dysfunction to the pathogenesis of chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019;33:782-790. doi:https://doi.org/10.1177/1945892419868588
- Holgate S. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233-1244. doi:https://doi.org/10.1016/j.jaci.2007.10.025
- Hong H, Liao S, Chen F. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy. 2020;75:2794-2804. doi:https://doi.org/10.1111/all.14526
- Mullol J, Picado C. Rhinosinusitis and nasal polyps in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:163-176. doi:https://doi.org/10.1016/j.iac.2012.11.002
- GINA Report 2024.
- Fokkens W, De Corso E, Backer V. EPOS2020/EUFOREA expert opinion on defining disease states and therapeutic goals in CRSwNP. Rhinology. 2024;62:287-298. doi:https://doi.org/10.4193/Rhin23.415
- Striz I, Golebski K, Strizova Z. New insights into the pathophysiology and therapeutic targets of asthma and comorbid chronic rhinosinusitis with or without nasal polyposis. Clin Sci. 2023;137:727-753. doi:https://doi.org/10.1042/CS20190281
- Matucci A, Bormioli S, Nencini F. Asthma and chronic rhinosinusitis: how similar are they in pathogenesis and treatment responses?. Int J Mol Sci. 2021;22. doi:https://doi.org/10.3390/ijms22073340
- De Corso E, Bilò M, Matucci A. Personalized management of patients with chronic rhinosinusitis with nasal polyps in clinical practice: a multidisciplinary Consensus Statement. JPM. 2022;12. doi:https://doi.org/10.3390/jpm12050846
- De Corso E, Pipolo C, Cantone E. Practical recommendations for managing severe chronic rhinosinusitis with nasal polyps in the era of biologics. Acta Otorhinolaryngol Ital. 2023;43:324-340. doi:https://doi.org/10.14639/0392-100X-N2422
- Fokkens W, Lund V, Bachert C. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74:2312-2319. doi:https://doi.org/10.1111/all.13875
- Bachert C, Han J, Wagenmann M. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: definitions and management. J Allergy Clin Immunol. 2021;147:29-36. doi:https://doi.org/10.1016/j.jaci.2020.11.013
- Fageeh Y, Basurrah M, Hakami K. Risk factors for recurrence of chronic rhinosinusitis with nasal polyps after endoscopic sinus surgery: a retrospective study. SMJ. 2023;44:1254-1259. doi:https://doi.org/10.15537/smj.2023.44.12.20230396
- Canonica G, Malvezzi L, Blasi F. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the Severe Asthma Network Italy (SANI) registry. Respir Med. 2020;166. doi:https://doi.org/10.1016/j.rmed.2020.105947
- Bilodeau L, Boulay M-E, Prince P. Comparative clinical and airway inflammatory features of asthmatics with or without polyps. Rhinology. 2010;48:420-425. doi:https://doi.org/10.4193/Rhino09.095
- Bleecker E, Al-Ahmad M, Bjermer L. Systemic corticosteroids in asthma: a call to action from World Allergy Organization and Respiratory Effectiveness Group. World Allergy Organ J. 2022;15. doi:https://doi.org/10.1016/j.waojou.2022.100726
- Bachert C, Hicks A, Gane S. The interleukin-4/interleukin-13 pathway in type 2 inflammation in chronic rhinosinusitis with nasal polyps. Front Immunol. 2024;15. doi:https://doi.org/10.3389/fimmu.2024.1356298
- Pelaia C, Pelaia G, Maglio A. Pathobiology of type 2 inflammation in asthma and nasal polyposis. JCM. 2023;12. doi:https://doi.org/10.3390/jcm12103371
- Caminati M, Caimmi C, Dama A. What lies beyond asthma control test: suggestions for clinical practice. J Asthma. 2016;53:559-562. doi:https://doi.org/10.3109/02770903.2015.1020386
- Marin C, Alobid I, López-Chacón M. Type 2 and non-type 2 inflammation in the upper airways: cellular and molecular alterations in olfactory neuroepithelium cell populations. Curr Allergy Asthma Rep. 2024;24:211-219. doi:https://doi.org/10.1007/s11882-024-01137-x
- Bachert C, Desrosiers M, Hellings P. The role of biologics in chronic rhinosinusitis with nasal polyps. J Pers Med. 2022;12. doi:https://doi.org/10.1016/j.jaip.2020.11.017
- De Corso E, Settimi S, Montuori C. How to manage recurrences after surgery in CRSwNP patients in the biologic era: a narrative review. Acta Otorhinolaryngol Ital. 2023;43:S3-S13. doi:https://doi.org/10.14639/0392-100X-suppl.1-43-2023-01
- Blanco-Aparicio M, Domínguez-Ortega J, Cisneros C. Consensus on the management of united airways disease with type 2 inflammation: a multidisciplinary Delphi study. Allergy Asthma Clin Immunol. 2023;19. doi:https://doi.org/10.1186/s13223-023-00780-9
- Mullol J, Maldonado M, Castillo J. Management of united airway disease focused on patients with asthma and chronic rhinosinusitis with nasal polyps: a systematic review. J Allergy Clin Immunol. 2022;10:2438-2447.e9. doi:https://doi.org/10.1016/j.jaip.2022.04.039
- Yii A, Tay T-R, Choo X. Precision medicine in united airways disease: a “treatable traits” approach. Allergy. 2018;73:1964-1978. doi:https://doi.org/10.1111/all.13496
- Caruso C, Colantuono S, Ciasca G. Different aspects of severe asthma in real life: role of Staphylococcus aureus enterotoxins and correlation to comorbidities and disease severity. Allergy. 2023;78:131-140. doi:https://doi.org/10.1111/all.15466
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1295 times
- PDF downloaded - 522 times



