Head and neck
Vol. 45: 111TH CONGRESS SIOECHCF - OFFICIAL REPORT 2025
Prognostic significance of surgical margins in open neck horizontal laryngectomy: a systematic review and meta-analysis
Abstract
Objective. Over the past two decades there has been a strategic shift in treating laryngeal cancer, with an increasing emphasis on preserving the anatomical structure and function of the larynx, even in cases of intermediate or advanced stages of disease. Open partial horizontal laryngectomies (OPHL) are widely adopted to spare the physiological functions of the larynx while achieving good oncological control. Positive, close or narrow surgical margins remain a critical prognostic factor, with their impact varying by tumour location and laryngeal subsite. This review examines the influence of positive margins on survival and the potential need for adjuvant treatments to optimise functional and oncological outcomes.
Methods. This study adhered to PRISMA guidelines. Using the PICOS framework, it included studies on adults with laryngeal squamous cell carcinoma treated by OPHL, focusing on survival and local control outcomes. A systematic search of PubMed, EMBASE, and Cochrane databases from 2000 to 2023 was conducted and eligible studies were selected based on comprehensive inclusion criteria and screening of references.
Results. The initial search yielded 675 articles from PubMed, 799 from EMBASE, and 33 from the Cochrane Library. After exclusions and duplicate removal, 57 full-text articles were reviewed, with 8 included for qualitative analysis and 7 for quantitative analysis. A total of 2,715 patients (age range, 16-87 years) were recruited across studies spanning from 2001 to 2021, all of which were retrospective. Among patients, 284 (10%) received neoadjuvant treatment and 627 (23%) underwent adjuvant therapy for positive margins, lymph node involvement and adverse pathological features. Seven studies assessed the association between margin status and recurrence, showing that close/positive margins significantly increased recurrence risk (OR 2.77, 95% CI 1.99-3.87, p < 0.01), with no publication bias detected.
Conclusions. This review highlights the challenges in defining and managing resection margins in OPHL for laryngeal cancer. While positive or close margins increase the risk of local recurrence, their effect on overall survival is unclear, emphasising the need for standardised protocols and individualised, multidisciplinary treatment planning.
Introduction
Over the past two decades there has been a strategic shift in treating laryngeal cancer, with an increasing emphasis on preserving the anatomical structure and function of the larynx, even in cases of intermediate or advanced stages of disease. This shift has positively influenced the broader adoption of conservative surgical techniques, such as open partial horizontal laryngectomies (OPHL), which aim to preserve respiratory, swallowing and phonatory functions without compromising oncological control 1.
Laryngeal cancer is generally characterised by a relatively favourable prognosis, especially when diagnosed at early stages 2. Furthermore, even in cases of intermediate or advanced disease, particularly in glottic cancers, the incidence of cervical lymph node metastasis remains relatively low 3. This makes conservative surgical techniques a viable therapeutic option when combined with a rigid selection process for both tumours and patients aimed at optimising functional and oncological outcomes.
The status of resection margins is a key aspect of oncological surgery and has been identified as a significant negative prognostic factor affecting local control 4,5. A universally accepted and consistent definition of negative, close and positive margins still needs refinement, considering that the classical definition of adequate margins (5 mm in final histopathology) has been challenged. Additionally, no clear prognostic definition exists when the involved margin is superficial or deep 6.
Previous studies have shown that positive or close margins, along with perineural invasion (PNI) and lympho-vascular invasion (LVI), may require adjuvant treatments such as postoperative radiotherapy 7-9. However, the prognostic significance of positive margins in open partial laryngeal surgery remains controversial with often conflicting results, particularly in cases where adjuvant radiotherapy is administered at different doses based on margin status.
OPHL comprises three different partial surgeries: OPHL type I (supraglottic laryngectomy), OPHL type II (supracricoid laryngectomy), and OPHL type III (supratracheal laryngectomy), each with specific extensions. These procedures involve removing a substantial portion of the larynx, up to four-fifths of the organ, making it challenging to achieve wide surgical margins. As a result, the margins obtained are often narrow, typically measuring only a few (2-3) mm 10.
Large-scale meta-analyses have demonstrated that the rates of local (LC) and locoregional control (LRC) associated with these techniques are excellent 12, suggesting that the clinical relevance of negative, close or positive margins may be less significant compared to other sites within the upper aerodigestive tract. Nevertheless, the impact of margins on oncological prognosis may significantly depend on the specific tumour location and laryngeal subsite. This “site dependency” has been observed in several series of partial laryngectomies, where surgical margins obtained in areas such as the glottis or subglottis may have different prognostic implications compared to other locations 6.
This systematic review explores the impact of positive margins on survival and oncological outcomes in patients with laryngeal cancer treated by OPHL. Our goal is to provide a more precise understanding of the need for adjuvant treatments in cases of positive or close margins, intending to minimise negative impact on the organ and maintaining functional preservation of the larynx.
Materials and methods
This study strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 13. Ethics committee approval or informed consent was not required, as all data were obtained from published literature.
The inclusion criteria for study selection were based on the PICOS framework: Population (P), adults with squamous cell carcinoma of the larynx; Intervention (I), OPHL; Comparator (C), none; Outcome (O), overall survival as the primary outcome, with LC, disease-free survival (DFS), disease-specific survival (DSS), and laryngectomy free survival (LFS) as secondary outcomes; Study design (S), retrospective. A systematic search was conducted for articles published between 2000 and 2023 in the PubMed, EMBASE, and Cochrane Library databases using the following combined search query: (“larynx” OR “laryngeal” OR “glottic” OR “supraglottic” OR “subglottic”) AND (“cancer” OR “carcinoma” OR “tumour” OR “neoplasm”) AND (“partial laryngectomy” OR “partial horizontal laryngectomy” OR “supraglottic partial laryngectomy” OR “supracricoid partial laryngectomy” OR “supratracheal partial laryngectomy” OR OPHL OR “supraglottic laryngectomy” OR “supracricoid laryngectomy” OR “supratracheal laryngectomy” OR cricohyoidoepiglottopexy OR cricohyoidopexy) NOT (Tors OR robotic OR TLM OR TOLM OR TOLMS OR “transoral surgery” OR microlaryngoscopy OR laser OR thyroid OR “salivary gland” OR “oral cavity”).
The full texts of relevant studies were reviewed for final selection. References from all the included studies were screened to identify any additional eligible papers. The most recent publication was selected if multiple articles from the same research group or centre described overlapping case series.
Eligibility assessment
Two authors (GA, MC) independently reviewed the studies identified in the initial search across the three databases. Articles were included only if both reviewers agreed. In case of disagreement, the full text was examined, and if uncertainty persisted, consultation with two expert authors (GS, EC) was sought.
Following PICOS guidelines, the study focused on patients with squamous cell carcinoma of the larynx who underwent OPHL as primary treatment. The inclusion criteria were as follows: 1) squamous cell carcinoma of the larynx confirmed by histopathological examination; 2) staging according to the American Joint Committee on Cancer (AJCC) 14; 3) patients surgically treated by OPHL, with or without neck dissection; 4) no prior treatment; 5) margins reported as negative/close/positive; 6) margin status correlated with at least one of the following oncological outcomes: LC, DSS, DFS, OS and the adjusted hazard ratio (aHR), which is based on margin status as a categorical variable (negative, close, positive). Studies without an aHR were excluded from the quantitative synthesis but included in the qualitative analysis.
The exclusion criteria were duplicate articles, book chapters, case reports, poster presentations, articles analysing head and neck tumours other than squamous cell carcinoma or squamous cell carcinomas originating from sites other than the larynx and articles not written in English.
OS was defined as the time from surgery to death or the last follow-up. LC was defined as the time from surgery to local recurrence or the last follow-up. DSS was defined as the time from surgery to cancer-related death or the last follow-up and DFS was the time until local, nodal or distant recurrence or the last follow-up.
Data extraction
The extracted data included the following: first author, year of publication, patient recruitment method, country, number of patients, age, gender, neck treatment, adjuvant treatment, TNM stage (AJCC 6th, 7th, or 8th editions), level of evidence (LOE), type of OPHL, margin status (positive, negative, close), aHR for LC, OS, DSS, DFS and follow-up duration.
Study quality assessment
The risk of bias was independently assessed by each reviewer according to the Reporting Recommendations for Tumour Prognostic Studies (REMARK) guidelines 15. Eight criteria were evaluated, each rated as either adequate (1) or inadequate (0): clearly defined inclusion and exclusion criteria, study type (prospective or retrospective), patient characteristics, tumour characteristics, margin status definition, study endpoints, follow-up duration and identification of patients lost to follow-up. Each study was assigned a total score from 0 to 8, with a score > 5 considered as globally adequate.
Statistical analysis
The meta-analysis was performed with IBM SPSS Statistics for Macintosh, Version 29.0 (IBM Corp., Armonk, NY, USA). To assess the associations between margin status (close and positive versus negative) and the endpoints, HRs with 95% CIs were pooled with a random-effects model. Heterogeneity between studies was assessed with the Cochran Q-statistic and I2 tests. Heterogeneity between studies was considered a p value of Q-test < 0.05 or I2 value >50%. Potential publication bias was estimated with Egger’s linear regression test, and a p value of < 0.05 was considered significant. Statistical significance was set at < 0.05, and all statistical tests were two-tailed.
Results
Literature search and study identification
A flowchart of the study selection process is shown in Figure 1. The initial search identified 675 articles from PubMed, 799 from EMBASE, and 33 from the Cochrane Library. After applying the exclusion criteria, non-English articles and irrelevant studies were discarded. After removing duplicates, 57 full-text articles were reviewed, with 8 included for qualitative analysis, and 7 for quantitative analysis.
Characteristics of studies included
A total of 2,715 patients were recruited, with individual study sample sizes ranging from 31 to 819. Eight studies reported the status of the margins as a categorical variable and used a cut-off value to dichotomise the data (Tab. I).
The publication period spanned 20 years (from 2001 to 2021), and the recruitment period was from 1972 to 2015. Patient ages ranged from 16 to 87 years. All studies included were retrospective (LOE IV) 15. Of the 2,715 patients, 284 (10%) underwent neoadjuvant treatment: 83 (29%) received radiotherapy, 101 (35%) chemotherapy, and 89 (31%) underwent previous surgery (transoral laser microsurgery, TOLMS).
All patients underwent OPHL. Following guidelines and institutional policies, 609 of 2,715 (22.4%) patients received adjuvant radiotherapy, while 18 (0.6%) chemoradiotherapy mainly due to positive margins, lymph node involvement and adverse pathological features. Follow-up periods ranged from 2 to 16 years. The patient and tumour characteristics of the eligible studies are listed in Tables II and III.
Quality assessment
After data collection, we evaluated the risk of bias in each study, independently adhering to the REMARK guidelines 15. REMARK involves 8 different domains. Each domain is considered adequate (1) or not (0):
- well-defined inclusion and exclusion criteria;
- nature of the study (prospective or retrospective);
- patient characteristics;
- tumour characteristics;
- margin status definition;
- study endpoints or outcomes;
- follow-up period;
- patients unavailable for statistical analysis identified (i.e., lost to follow-up).
We gave each study a total score from 0 to 8, indicating the lowest and highest quality, respectively. A total score > 5 was considered globally adequate. The quality of the studies ranged from 5 to 8. The median score was 6.6.
Meta-analysis
Seven studies were eligible to investigate the association between margin status and the development of recurrence. Although the studies appear homogeneous (Q-test p = 0.776, I2 = 0%), subsequent analyses were still carried out using the random-effects model because of the limited number of studies. The results of the meta-analysis indicated that there is a role of involvement of surgical margins (close/positive versus negative) in the development of recurrences (OR 2.77, 95% CI 1.99 to 3.87, p < 0.01) (Fig. 2). Egger’s test showed no substantial publication bias (p = 0.201).
Discussion
In recent decades, laryngeal preservation has emerged as a primary goal in the management of laryngeal cancer, using both non-surgical and surgical strategies to maintain organ function while ensuring adequate oncological control. This approach was strengthened by the 2017 American Society of Clinical Oncology guidelines, which recommend laryngeal preservation as the initial treatment strategy for T1-T2 tumours 16. Although rarely reported, carefully selected cases of primary T3-T4 tumours may also be eligible for organ-preserving procedures, such as OPHLs 1. However, the limited anatomical space within the larynx presents challenges in achieving optimal resection margins in these procedures, with significant implications for both LC and functional outcomes 4,11.
OPHL techniques, now recognised as standardised procedures, have proven effective for complex tumours that are challenging to manage with minimally invasive approaches like TOLMS, particularly in cases with vertical commissure involvement or suspected posterior paraglottic space extension 17. OPHL procedures offer high freedom from laryngectomy (FFL) rates and favourable oncological outcomes for intermediate-staged tumours 18,19, although sometimes at the expense of good vocal quality. The OPHL classification of the European Laryngological Society 10 provided a structured framework, correlating the extent of resection with minimal achievable margins, generally within a few millimetres, due to the confined laryngeal anatomy 11,20.
A primary consideration in laryngeal surgery is the adequacy of resection margins, which is critical for effective local control. Four to five mm margins are generally considered adequate for open resections 4,7, while ≤ 2 mm is typically accepted for endoscopic procedures of glottic tumours 21. Despite these guidelines, no universal agreement categorises margins as positive, close or negative. Several studies attempted to address this gap; for example, Session 7 and Gallo4 classified margins greater than 5 mm as negative, those under 5 mm as close and tumour-involved margins as positive. Gallo4 and Nakayama 6 additionally proposed a distinction between dysplasia and invasive carcinoma at one margin, suggesting a histological gradient where dysplasia at one superficial edge of resection might signify a “close” margin. In contrast, in situ or invasive carcinoma represents a truly “positive” margin. Such a classification could allow for more tailored postoperative decisions, reserving additional treatments only for cases with confirmed positive margins.
This systematic review highlights a wide range of positive margin incidences in laryngeal cancer resections, varying from 3% to 60%, with many studies reporting a 10% baseline. The study from Lee 22 reported a positive margin rate of 4%, while Gallo 4 observed an incidence of 16%. Discrepancies can arise from differences in defining a tumour-positive margin and in patient cohorts, with some series focusing more on advanced T3-T4 tumours. For example, Kligerman et al. 23 found a 24% positive margin rate among T3-T4 tumours.
This meta-analysis assessed the prognostic value of positive resection margins and found their impact on OS, DFS, and DSS to be inconclusive, partly because different studies refer to positive, close or negative margins without consistently specifying the cutoff values or the techniques used (e.g., frozen sections on the margins of the resection bed vs. margins on the specimen). However, positive or close margins significantly affected LC. Several factors may explain the heterogeneity in these findings, including diversity in T categories and laryngeal subsites, inconsistent differentiation between superficial and deep margins, and variations in how positive margins are managed (e.g., close follow-up versus adjuvant radiotherapy). Moreover, some studies do not specify whether positive margins were single or multiple, superficial or deep, complicating the assessment of a reliable link between margin status and prognostic outcomes.
Saraniti et al. 24, in a study analysing outcomes of a series of 139 patients undergoing total or partial laryngectomies and comparing them with those reported in the literature, concluded that such comparisons are not feasible. They emphasised that, to investigate the prognostic value of resection margins, a meta-analysis should be conducted using standardised definitions of resection margins, consistent methodologies and uniform postoperative treatments.
In 253 OPHL type IIa or b procedures, Gallo et al. 4 identified a significant difference in local recurrence rates between patients with positive and negative margins (22.5% vs 6.1%, p < 0.05), associating positive or close margins with higher recurrence. Nakayama et al. 6 reported similar findings in OPHL type II, where patients with positive or dysplastic margins experienced slightly higher rates of recurrence (p < 0.05). In a cohort of 118 OPHL type IIa and b, some of whom received induction chemotherapy, Dufour et al. 25 found a significant relationship between positive margins and local recurrence in both univariate (p < 0.001) and logistic regression models (p < 0.008), highlighting the importance of achieving clear margins. Crosetti et al. 26, in a larger sample of OPHL type I-III, also found margin status to be a significant predictor of local recurrence in univariate analysis (p < 0.001). However, this association did not hold in multivariate testing, indicating that margins, while critical, are only one of many prognostic factors.
These findings suggest that, although positive or close margins are associated with increased local recurrence, clear margins do not always guarantee LC. Residual tumor may persist in non-resected tissue, even when margins appear clean. Additionally, the anatomical complexity of the larynx poses challenges in specimen handling. Sampling errors during specimen preparation, especially in frozen sections, can lead to misinterpretation of margin status.
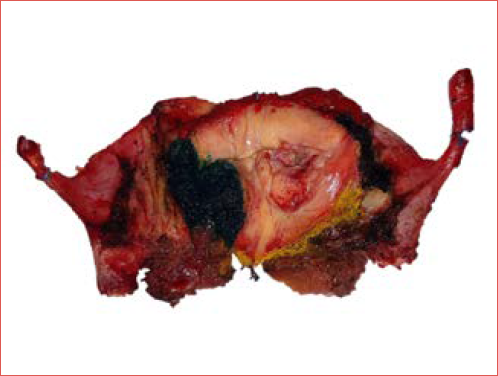
Histologic evaluation of surgical margins serves as an essential quality control in assessing resection adequacy (Cover figure). However, accurately assessing margins in the intricate laryngeal anatomy is challenging due to factors like tissue shrinkage, electrosurgical damage, and sampling variability. Thermal damage from electrosurgical tools during OPHL can obscure tumour-free boundaries, resulting in false positives or negatives.
A standardised approach to specimen processing and interpretation is crucial, with close attention to technical details like tissue shrinkage, sampling methodology and fixation protocols 11. Centres with advanced diagnostic imaging (e.g., MRI with diffusion-weighted imaging, HDTV-enhanced narrow band imaging) may consider a conservative follow-up for patients with single close or positive superficial margins, reserving adjuvant therapy for cases with additional adverse features, such as LVI, PNI 26 or advanced T-categories.
Different approaches exist for managing positive margins: while some authors 7 recommend adjuvant radiotherapy in all positive cases, others 4 suggest a more conservative follow-up-centred strategy to avoid functional impairment. In OPHL cases, a second-look (always possible in cases treated by TOLMS) cannot be performed to verify residual disease, making completion laryngectomy an option upon confirmation of positive margins in the final pathology report. However, this approach carries significant psychological and ethical considerations, as patients often reject total laryngectomy, especially given the literature’s unclear impact on DFS.
In evaluating margin prognostic value, additional adverse pathological features – such as advanced T-categories and lymph node(s) positivity (N+) – significantly affect outcomes 26. Only tertiary centres experienced in OPHL follow-up and equipped with advanced diagnostic tools should be considered for a conservative approach with positive margins. Moreover, studies rarely specify whether margins are single or multiple, superficial, deep or extra-laryngeal, which would impact adjuvant therapy decisions. Recurrence patterns after OPHL are frequently loco-regional, though it is often unclear whether recurrence is related to the T or N category 26. These differences may reflect variations in lymphatic drainage within the glottic region, potentially reducing loco-regional recurrence risks and enhancing DSS.
Typically, adjuvant treatment consists of radiotherapy, sometimes with concurrent chemotherapy in cases with additional risk factors such as multiple lymph nodes positivity and/or extranodal extension. This aims to mitigate the negative impact of positive margins on LC, FFL and laryngo-oesophageal dysfunction free survival (LEDFS). Treatment approaches for positive margins following OPHL vary widely, with Nakayama 6 recommending adjuvant radiotherapy for only 20% of close/positive cases. At the same time, Dufour 25 and Session 7 suggest broader radiotherapy applications based on pathological staging. Crosetti 26 and Damiani 8 gave indications for adjuvant radiotherapy in all positive cases. At the same time, Gallo 4 selectively recommends it for only 25% of positive cases based on location (deep or posterior), adopting a close monitoring approach for others.
Adjuvant irradiation can cause laryngeal radionecrosis 27, post-radiation oedema and fibrosis, impacting decannulation, swallowing and recurrence evaluation. Muscatello et al. 9, in a multi-institutional study of 130 OPHL type II-III treated by adjuvant (chemo)RT, reported a 5-year LEDFS of 85%. However, 13% of patients remained tracheostomy- and 3% gastrostomy-dependent at the last follow-up. Therefore, decisions on adjuvant radiotherapy should be carefully balanced and guided by the tumour board, considering both pTN staging and adverse pathological features to prevent overtreatment, especially in intermediate-stage tumours where single-treatment oncological success is achievable.
Conclusions
This review illustrates the challenges and variability in defining and managing resection margins in OPHL for laryngeal cancer. Although positive or close margins are linked to an increased risk of local recurrence, their impact on OS remains unclear. Further multicentre, prospective studies are essential to standardise definitions for margins and treatment protocols while prioritising oncological efficacy and functional preservation. Based on emerging evidence from the literature, it can be stated that, regarding margins on the specimen, a disease-free margin of at least 2-3 mm should be considered sufficient and safe, a disease-free margin of 1-2 mm should be considered close, and all cases presenting intraepithelial or invasive disease at the resection edge should be considered truly positive. Due to the complex interpretation of margin status, individualised, multidisciplinary adjuvant treatment planning – especially in tertiary centres with advanced follow-up tools – remains vital for optimising patient outcomes.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
EC: conception and design of the study, surgeon, writing and editing the manuscript; GS: conception and design of the study, surgeon, editing the manuscript; GA, MC, GP: data collection, surgeon; AG: conception and design of the study, reviewing the manuscript, surgeon; AB, GP, reviewing the manuscript, surgeon; AES: statistical; MDV: conception and design of the study, reviewing the manuscript, surgeon.
Ethical consideration
Not applicable.
History
Received: January 21, 2025
Accepted: March 10, 2025
Figures and tables
Figure 1. PRISMA flowchart.
Figure 2. Forest plot of the meta-analysis regarding the association between margin status and recurrence.
| Study | Year | Country | Study period | Prospective retrospective cohort study | Endpoint | Follow-up time |
|---|---|---|---|---|---|---|
| Nakayama 11 | 2011 | Japan | 1997-2009 | Retrospective | LC | Not specified |
| Dufour 25 | 2004 | France | 1972-1997 | Retrospective | LC | 5 years |
| Crosetti 26 | 2019 | Italy | 1995-2014 | Retrospective | OS, DSS, DFS, FFL, LFS, LEDFS | 2-16.4 years |
| Yilmaz 28 | 2001 | Turkey | Not specified | Retrospective | LC | 5-16 years |
| Damiani 8 | 2021 | France | 1999-2015 | Retrospective | OS | 16 years |
| Gallo 4 | 2004 | Italy | 1984-2001 | Retrospective | OS | 4.3 years |
| Sessions 7 | 2005 | USA | 1955-1999 | Retrospective | DSS | 5 years |
| Soundry 29 | 2010 | Israel | 1996-2005 | Retrospective | OS | 4.7 years |
| LC: local control; OS: overall survival; DSS: disease-specific survival; DFS: disease-free survival; FFL: freedom from laryngectomy; LFS: laryngectomy-free survival; LEDFS: laryngo-esophageal dysfunction-free survival. | ||||||
| Study | No. of patients | Median age | Previous treatment | Type of surgery on the T | Type of surgery on the N | TNM stage |
|---|---|---|---|---|---|---|
| Nakayama 11 | 61 | 62 (42-76) | 26/61 (43%) RT | OPHL IIa 56 (91.8%) | Not indicated | pT1-T2 23/61 (38%) |
| (59M/2F) | OPHL IIb 4 (6.5%) | pT3-T4 38/61 (62%) | ||||
| TL 1 (1.6%) | ||||||
| Dufour 25 | 118 (109M/9F) | 58 (35-83) | 100/118 (85%) ICT | OPHL IIa 37 (31.3%) | ND 99/118 (84%) | cT3 118 (100%) |
| OPHL IIb 81 (68.7%) | ||||||
| Crosetti 26 | 819 (755M/64F) | 60 (16-87) | 140/819 (17%) | OPHL IIa 159 (19.4%) | ND 704/819 | pT2 236 (28.8%) |
| OPHL IIa+ARY 354 (43.2%) | (85.9%) | pT3 437 (53.4%) | ||||
| TOLMS 61/140 (43.6%) | OPHL IIb 46 (5.6%) | Ipsilateral | pT4 146 (17.8%) | |||
| RT 39/140 (27.9%) | OPHL IIb+ARY 138 (16.8%) | 606 (86.1%) | ||||
| open cordectomy 21/140 (15%) | OPHL IIIa 10 (1.2%) | bilateral | ||||
| OPHL 7/140 (5%) | OPHL IIIa +CAU 99 (12.1%) | 98 (13.9%) | ||||
| ICT 1/140 (0.7%) | OPHL IIIb 5 (0.6%) | |||||
| OPHL IIIb+CAU 8 (1%) | ||||||
| Yilmaz 28 | 21 positive | Not indicated | None | TL 9/21(42.9%) | Not indicated | pT1 5/21 (24%) |
| margins/714 TL/PL | SPL 5/21 (23.8%) | pT2 10/21 (48%) | ||||
| (714 M/0 F) | VL 2/21 (9.5%) | pT3 1/21 (4%) | ||||
| Endolaryngeal PL 5/21 (23.8%) | pT4 5/21 (24%) | |||||
| Damiani 8 | 68 | 57 (39-72) | None | OPHL I 68 (100%) | ND 68 (100%) | pT1 3/68 (4%) |
| (55 M/13 F) | Ipsilateral | pT2 23/68 (34%) | ||||
| 16/68 (24%) | pT3 42/68 (62%) | |||||
| Bilateral 52/68 (76%) | ||||||
| Gallo 4 | 253 | 58 (35-77) | None | OPHL IIb 180/253 (71%) | ND 125 (49.4%) | cT1 26/253 (10.3%) |
| (234 M/19 F) | OPHL IIa 73/253 (29%) | Ipsilateral 103 (82.4%) | cT2 148/253 (58.5%) | |||
| Bilateral 22 (17.6%) | cT3 64/253 (23.5%) | |||||
| cT4 15/253 (5.9%) | ||||||
| Sessions 7 | 653 | 28-81 | None | OPHL I 403/653 (61.7%) | ND 465/653 (71%) | cT1 154/653 (23.6%) |
| (500 M/153 F) | TL 206/653 (31.5%) | cT2 231/653 (35.4%) | ||||
| RT 44/653 (6.8%) | cT3 170/653 (26%) | |||||
| cT4 98/653 (15%) | ||||||
| Soundry 29 | 29 | Not indicated | RT 18/29 (62%) | OPHL 15/29 (52%) | Not indicated | cT3-T4 |
| FLL 10/29 (34%) | Not specified | |||||
| Extended SP 4/29 (14%) | ||||||
| M: male; F: female; RT: radiotherapy; OPHL: open partial horizontal laryngectomy; TL: total laryngectomy;ND: neck dissection; TOLMS: transoral laser microsurgery; ICT: immunochemotherapy; CAU: crico arytenoid unit; ARY: arytenoid; PL: partial laryngectomy; SPL: supraglottic laryngectomy; VL: vertical laryngectomy; FLL: frontolateral laryngectomy; SP: supraglottic laryngectomy. | ||||||
| Study | Margin status | Adjuvant treatment | Treatment of recurrence | Oncologic results | HR value for endpoints |
|---|---|---|---|---|---|
| Nakayama 11 | Negative 36/61 (59%) | RT 5/61 (8.2%) | TL + CT 3/4 (75%) | Local recurrence 4/61 (7%) | Local recurrence vs dysplasia on margin |
| Dysplasia 18/61(30%) | 1 (25%) pt refused | Negative margins → local recurrence 2/36 (6%) | p < 0.05 | ||
| Positive 7/61 (11%) | Dysplasia → local recurrence 1/18 (6%) | ||||
| Positive margins → local recurrence 1/7 (14%) | |||||
| Dufour 25 | Positive 3/118 (2.5%) | RT 24/118 (20%) | TL 9/9 (100%) | Local recurrence 9/118 (7.6%) | Univariate analysis: margins of resections vs local recurrence (p < 0.001) |
| Close 3/118 (2.5%) | TL+PORT 3/9 (33.3%) | Positive margins → local recurrence 2/3 (66.7%) | |||
| Negative 112/118 (94.9%) | Close margins → local recurrence 0/3 (0%) | In regression model positive margin vs risk of local recurrence (p = 0.008) | |||
| Negative margins → local recurrence 7/112 (6.2%) | |||||
| Crosetti 26 | Positive 68/819 (8.3%) | 95/819 (11.6%) | Not indicated | Local recurrence 108/819 (13.2%) | Univariate analysis: status of margin vs recurrence (p = 0.013) |
| Close 43/819 (5.3%) | 94/95 (99%) RT | Positive margins → local recurrence 13/68 (19%) | |||
| Negative 708/819 (86.4%) | 1/95 (1%) CT | Close margins → local recurrence 14/43 (32.5%) | Logistic regression model: status of margin vs recurrence (p = 0.263) | ||
| Negative margins → local recurrence 81/708 (11.4%) | |||||
| Yilmaz 28 | Negative 536/714 (75%) | PORT 19/21 (90.4%) | Positive margins → local recurrence 9/21 (43%) | Locoregional recurrence vs free margin vs positive margin (124/412 vs 9/10; p = 0.02) | |
| Positive 21/714 (3%) | Negative margin → locoregional recurrence 124/536 (23%) | ||||
| Damiani 8 | Negative 58/68 | 27/68 (40%) PORT | 1/6 (16.7%) salvage surgery | Local recurrence 6/68 (8.8%) | Univariate analysis: OS vs positive margin (p = 0.01) |
| Positive 10/68 (15%) | 17/68 (25%) CCRT | 1/6 (16.7%) TL | Negative margins → local recurrence 5/58 (9%) | Multivariate analysis: positive margin vs OS (HR 2.47; 95% CI, 1.06-5.76; p = 0.037) | |
| 2/6 (33.3%) CCRT | Positive margins → local recurrence 1/10 (10%) | ||||
| 2/6 (33.3%) CT | |||||
| Gallo 4 | Negative 213/253 (84%) | 10 positive margins/40 (25%) PORT | TL 11/22 (50.2%) | Locoregional recurrence 22/253 (8.7%) | Univariate analysis: recurrence vs status of resection margins (p = 0.002) |
| Gallo 4 | Positive 40/253 (16%) | TL +PORT 2/22 (9%) | Negative margins → locoregional recurrence 13/213 (6.1%) | ||
| (Dysplasia 27.5%, invasive ca 72.5%) | PORT 2/22 (9%) | Negative margins → locoregional recurrence 13/213 (6.1%) | |||
| NearTL 2/22 (9%) | |||||
| CCRT 1/22 (4.6%) | |||||
| CT 1/22 (4.6%) | |||||
| ND 2/22 (9%) | |||||
| ND+PORT 1/22 (4.6%) | |||||
| Sessions 7 | Negative (> 5 mm) | 423/653 (64.7%) PORT | Not indicated | 212/653 (32.5%) recurrence | DSS negative margins 70.3% |
| Close (< 5 mm) 52/653 (9.1%) | DSS negative margins 72% | ||||
| Positive 76/653 (13.3%) | DSS close margins 66% | ||||
| DSS positive margins 51.5% (p = 0.0094) | |||||
| Soundry 29 | Positive 12/29 (41%) | 7/8 (87.5%) PORT with positive margin post primary surgery | 9/9 (100%) TL | Local recurrence 9/29 (31%) | DFS 5 y positive margin vs negative margin (67% vs 68%; p = 0.287) |
| (66% after primary surgery, 33.4% after RT + surgery) | 1/8 (12.5%) refused | Positive margins → recurrence 5/12 (41.6%) (12.5% after primary surgery, 100% after RT+surgery) | OS 5 y positive surgical margin vs negative (73% vs 94%; p = 0.051) | ||
| Negative 17/29 (59%) | Negative margin → recurrence 4/17 (23.5%) (0% after primary surgery, 28.5% after RT+surgery) | On Cox regression positive margin status is an independent prognostic factor for decreased survival (HR 6.349; p = 0.035) | |||
| (17.6% after primary surgery, 82.3% after RT+surgery) | |||||
| RT: radiotherapy; TL: total laryngectomy; CT: chemotherapy; PORT: post-operative radiotherapy; OS: overall survival; CCRT: concomitant chemoradiotherapy; HR: hazard ratio; ND: neck dissection; DSS: disease-specific survival; DFS: disease-free survival. | |||||
References
- Crosetti E, Fantini M, Bertotto I. Current status of partial laryngeal surgery for advanced laryngeal cancer: when and why?. Curr Oncol Rep. 2024;26:614-624. doi:https://doi.org/10.1007/s11912-024-01516-7
- Guimarães A, Dedivitis R, Matos L. Comparison between transoral laser surgery and radiotherapy in the treatment of early glottic cancer: a systematic review and meta-analysis. Sci Rep. 2018;8:1-7. doi:https://doi.org/10.1038/s41598-018-30218-x
- Succo G, Crosetti E, Bertolin A. Benefits and drawbacks of open partial horizontal laryngectomies, Part A: early – to intermediate-stage glottic carcinoma. Head Neck. 2016;38:E333-340. doi:https://doi.org/10.1002/hed.23997
- Gallo A, Manciocco V, Tropiano M. Prognostic value of resection margins in supracricoid laryngectomy. Laryngoscope. 2004;114:616-621. doi:https://doi.org/10.1097/00005537-200404000-00005
- Blanch J, Vilaseca I, Bernal-Sprekelsen M. Prognostic significance of surgical margins in transoral CO2 laser microsurgery for T1-T4 pharyngo-laryngeal cancers. Eur Arch Otorhinolaryngol. 2007;264:1045-1051. doi:https://doi.org/10.1007/s00405-007-0320-2
- Nakayama M, Okamoto M, Iwabuchi K. Clinical significance of intraoperative surgical margin study in supracricoid laryngectomy. Auris Nasus Larynx. 2011;38:261-265. doi:https://doi.org/10.1016/j.anl.2010.07.007
- Sessions D, Lenox J, Spector G. Supraglottic laryngeal cancer: analysis of treatment results. Laryngoscope. 2005;115:1402-1410. doi:https://doi.org/10.1097/01.MLG.0000166896.67924.B7
- Damiani M, Mercante G, Abdellaoui M. Prognostic features in intermediate-size supraglottic tumors treated with open supraglottic laryngectomy. Laryngoscope. 2021;131:E1980-1986. doi:https://doi.org/10.1002/lary.29367
- Muscatello L, Piazza C, Peretti G. Open partial horizontal laryngectomy and adjuvant (chemo)radiotherapy for laryngeal squamous cell carcinoma: results from a multicenter Italian experience. Eur Arch Otorhinolaryngol. 2021;278:4059-4065. doi:https://doi.org/10.1007/s00405-021-06651-6
- Succo G, Peretti G, Piazza C. Open partial horizontal laryngectomies: a proposal for classification by the working committee on nomenclature of the European Laryngological Society. Eur Arch Otorhinolaryngol. 2014;271:2489-2496. doi:https://doi.org/10.1007/s00405-014-3024-4
- Nakayama M, Holsinger C, Okamoto M. Clinicopathological analyses of fifty supracricoid laryngectomized specimens: evidence base supporting minimal margins. ORL J Otorhinolaryngol Relat Spec. 2009;71:305-311. doi:https://doi.org/10.1159/000261836
- Paleri V, Thomas L, Basavaiah N. Oncologic outcomes of open conservation laryngectomy for radiorecurrent laryngeal carcinoma. Cancer. 2011;117:2668-2676. doi:https://doi.org/10.1002/cncr.25831
- McInnes M, Moher M, Thombs B. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies. JAMA. 2018;319:388-396. doi:https://doi.org/10.1001/jama.2017.19163
- Amin M, Edge S, Greene F. AJCC Cance Staging Manual. Springer; 2017.
- Altman D, McShane L, Sauerbrei W. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9. doi:https://doi.org/10.1371/journal.pmed.1001216
- Forastiere A, Ismaila N, Lewin J. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36:1143-1169. doi:https://doi.org/10.1200/JCO.2017.75.7385
- Piazza C, Filauro M, Paderno A. Three-dimensional map of isoprognostic zones in glottic cancer treated by transoral laser microsurgery as a unimodal treatment strategy. Front Oncol. 2018;8. doi:https://doi.org/10.3389/fonc.2018.00175
- Succo G, Crosetti E, Bertolin A. Benefits and drawbacks of open partial horizontal laryngectomies, Part B: intermediate and selected advanced stage laryngeal carcinoma. Head Neck. 2016;38:E649-E657. doi:https://doi.org/10.1002/hed.24064
- Del Bon F, Piazza C, Lancini D. Open partial horizontal laryngectomies for T3-T4 laryngeal cancer: prognostic impact of anterior vs. posterior laryngeal compartmentalization. Cancers (Basel). 2019;11. doi:https://doi.org/10.3390/cancers11030289
- Succo G, Crosetti E, Bertolin A. Treatment of T3-T4a laryngeal cancer by open partial horizontal laryngectomies: prognostic impact of different pT subcategories. Head Neck. 2018;40:1897-1908. doi:https://doi.org/10.1002/hed.25176
- Iandelli A, Gabella G, Marchi F. The impact of margins in laryngeal cancer patients treated with transoral laser microsurgery: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2024;281:4485-4494. doi:https://doi.org/10.1007/s00405-024-08610-3
- Lee J. Detection of residual carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx: a study of surgical margins. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:49-53.
- Kligerman J, Olivatto L, Lima R. Elective neck dissection in the treatment of T3/T4 N0 squamous cell carcinoma of the larynx. Am J Surg. 1995;170:436-439. doi:https://doi.org/10.1016/S0002-9610(99)80324-9
- Saraniti C, Speciale R, Gallina S. Prognostic role of resection margin in open oncologic laryngeal surgery: survival analysis of a cohort of 139 patients affected by squamous cell carcinoma. Braz J Otorhinolaryngol. 2022;16:1-23. doi:https://doi.org/10.1016/j.bjorl.2018.04.012
- Dufour X, Hans S, De Mones E. Local control after supracricoid partial laryngectomy for “advanced” endolaryngeal squamous cell carcinoma classified as T3. Arch Otolaryngol Head Neck Surg. 2004;130:1092-1099. doi:https://doi.org/10.1001/archotol.130.9.1092
- Crosetti E, Bertolin A, Molteni G. Pattern of recurrence after open partial horizontal laryngectomy types II and III: univariate and logistic regression analysis of risk factors. Acta Otorhinolaryngol Ital. 2019;39:235-243. doi:https://doi.org/10.14639/0392-100X-2409
- Melo G, Souza P, Bastos Filho L. Laryngeal chondroradionecrosis following radiotherapy. Rev Col Bras Cir. 2017;44:374-382. doi:https://doi.org/10.1590/0100-69912017004012
- Yilmaz T, Turan E, Gursel B. Positive surgical margins in cancer of the larynx. Eur Arch Otorhinolaryngol. 2001;258:188-191. doi:https://doi.org/10.1007/s004050100325
- Soudry E, Hadar T, Shvero J. The impact of positive resection margins in partial laryngectomy for advanced laryngeal carcinomas and radiation failures. Clin Otolaryngol. 2010;35:402-408. doi:https://doi.org/10.1111/j.1749-4486.2010.02188
Downloads
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright
Copyright (c) 2025 Società Italiana di Otorinolaringoiatria e chirurgia cervico facciale
How to Cite
- Abstract viewed - 1204 times
- PDF downloaded - 418 times



